当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced As(III) Sequestration Using Sulfide-Modified Nano-Scale Zero-Valent Iron with a Characteristic Core–Shell Structure: Sulfidation and As Distribution
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2018-01-30 00:00:00 , DOI: 10.1021/acssuschemeng.7b02787 Deli Wu 1, 2 , Shuhan Peng 1, 2 , Kaili Yan 1, 2 , Binbin Shao 1, 2 , Yong Feng 3 , Yalei Zhang 1, 2
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2018-01-30 00:00:00 , DOI: 10.1021/acssuschemeng.7b02787 Deli Wu 1, 2 , Shuhan Peng 1, 2 , Kaili Yan 1, 2 , Binbin Shao 1, 2 , Yong Feng 3 , Yalei Zhang 1, 2
Affiliation
|
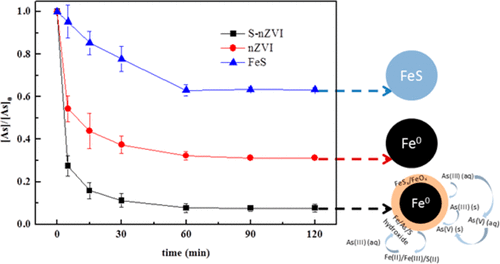
Sulfide-modified nano-scale zero-valent iron (S-nZVI) was synthesized and employed for the removal of aqueous As(III). The structure and removal performance of S-nZVI was investigated and compared with that of pristine nZVI. S-nZVI has an optimal As removal capacity of 240 mg/g, which is much higher than that of nZVI. The sulfidation of nZVI also enhanced the As(III) removal rate, and the enhancement largely depended on the S/Fe molar ratio. The optimum pH for As(III) removal with S-nZVI was in a broad range from 3 to 8. Transmission electron microscopy (TEM) with energy-dispersive X-ray spectroscopy (EDS), scanning electron microscopy (SEM) with EDS, and X-ray photoelectron spectroscopy (XPS) were employed to characterize the S-nZVI before and after reacting with As(III). The results demonstrated that S-nZVI had a unique core–shell structure. Sulfur was incorporated into the shell of S-nZVI, and the thickness of the surface layer increased from 5 nm to approximately 30 nm, which suggested that more As(III) could be sequestered by the nanoparticles. Therefore, better As(III) removal was observed with the increased S/Fe molar ratio. The As distribution in solid phase was used to describe the As(III) removal mechanism, and the results revealed that As(III) removal is a complex process that includes surface adsorption and coprecipitation. Sulfidation promoted the precipitation process and inhibited outer-sphere complexation, which led to enhanced As removal. Oxygen impaired the structure of S-nZVI and generated oxidants via iron sulfide transformations, which drove theAs(III) oxidation and contributed to the total As removal. The large As(III) removal capability and chemical stability of S-nZVI show its potential as an effective and environmentally friendly material for As(III) removal from aqueous solutions.
中文翻译:

使用具有特征核-壳结构的硫化物修饰的纳米级零价铁增强As(III)螯合:硫化和As分布
合成了硫化物改性的纳米级零价铁(S-nZVI),并用于去除As(III)水溶液。研究了S-nZVI的结构和去除性能,并与原始nZVI进行了比较。S-nZVI的最佳As去除能力为240 mg / g,远高于nZVI。nZVI的硫化也提高了As(III)的去除率,而提高的程度主要取决于S / Fe摩尔比。用S-nZVI去除As(III)的最佳pH在3到8的宽范围内。透射电子显微镜(TEM)和能量色散X射线光谱(EDS),扫描电子显微镜(SEM)和EDS,用X射线光电子能谱(XPS)表征与As(III)反应前后的S-nZVI。结果表明,S-nZVI具有独特的核-壳结构。硫被掺入S-nZVI的壳中,表面层的厚度从5 nm增加到大约30 nm,这表明纳米颗粒可以螯合更多的As(III)。因此,随着S / Fe摩尔比的增加,可以更好地去除As(III)。固相中As的分布用于描述As(III)的去除机理,结果表明As(III)的去除是一个复杂的过程,包括表面吸附和共沉淀。硫化促进了沉淀过程并抑制了外球络合,从而提高了As的去除率。氧气破坏了S-nZVI的结构,并通过硫化铁转化生成了氧化剂,从而推动了As(III)的氧化并促进了As的总去除。
更新日期:2018-01-30
中文翻译:

使用具有特征核-壳结构的硫化物修饰的纳米级零价铁增强As(III)螯合:硫化和As分布
合成了硫化物改性的纳米级零价铁(S-nZVI),并用于去除As(III)水溶液。研究了S-nZVI的结构和去除性能,并与原始nZVI进行了比较。S-nZVI的最佳As去除能力为240 mg / g,远高于nZVI。nZVI的硫化也提高了As(III)的去除率,而提高的程度主要取决于S / Fe摩尔比。用S-nZVI去除As(III)的最佳pH在3到8的宽范围内。透射电子显微镜(TEM)和能量色散X射线光谱(EDS),扫描电子显微镜(SEM)和EDS,用X射线光电子能谱(XPS)表征与As(III)反应前后的S-nZVI。结果表明,S-nZVI具有独特的核-壳结构。硫被掺入S-nZVI的壳中,表面层的厚度从5 nm增加到大约30 nm,这表明纳米颗粒可以螯合更多的As(III)。因此,随着S / Fe摩尔比的增加,可以更好地去除As(III)。固相中As的分布用于描述As(III)的去除机理,结果表明As(III)的去除是一个复杂的过程,包括表面吸附和共沉淀。硫化促进了沉淀过程并抑制了外球络合,从而提高了As的去除率。氧气破坏了S-nZVI的结构,并通过硫化铁转化生成了氧化剂,从而推动了As(III)的氧化并促进了As的总去除。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号