当前位置:
X-MOL 学术
›
Cell Death Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sphingomyelin synthase 2 promotes an aggressive breast cancer phenotype by disrupting the homoeostasis of ceramide and sphingomyelin.
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-02-15 , DOI: 10.1038/s41419-019-1303-0 Kehong Zheng 1, 2 , Zetao Chen 3, 4 , Haizhan Feng 1 , Ying Chen 1 , Cheng Zhang 1 , Jinlong Yu 1 , Yunfeng Luo 1 , Liang Zhao 3, 4 , Xiancheng Jiang 5 , Fujun Shi 1
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-02-15 , DOI: 10.1038/s41419-019-1303-0 Kehong Zheng 1, 2 , Zetao Chen 3, 4 , Haizhan Feng 1 , Ying Chen 1 , Cheng Zhang 1 , Jinlong Yu 1 , Yunfeng Luo 1 , Liang Zhao 3, 4 , Xiancheng Jiang 5 , Fujun Shi 1
Affiliation
|
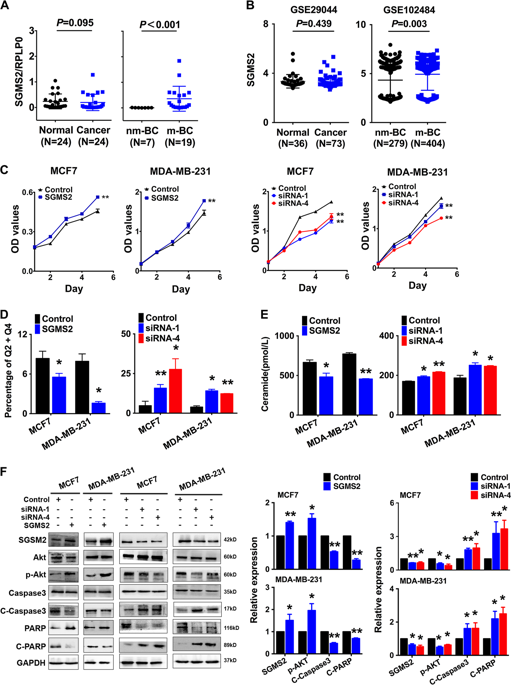
Breast cancer is the most common type of carcinoma in women worldwide, but the mechanisms underlying tumour development and progression remain unclear. Sphingomyelin synthase 2 (SGMS2) is a crucial regulator involved in ceramide (Cer) and sphingomyelin (SM) homoeostasis that is mostly studied for its role in lipid metabolism. Our primary study indicated that high SGMS2 expression is associated with breast cancer metastasis. Gain- and loss-of-function assays in vitro and in vivo revealed that SGMS2 promotes cancer cell proliferation by suppressing apoptosis through a Cer-associated pathway and promotes cancer cell invasiveness by enhancing epithelial-to-mesenchymal transition (EMT) initiation through the TGF-β/Smad signalling pathway. Further study determined that SGMS2 activated the TGF-β/Smad signalling pathway primarily by increasing TGF-β1 secretion, which was likely associated with aberrant expression of SM. Thus, our findings indicate that SGMS2-mediated activation of the TGF-β/Smad signalling pathway is important in breast cancer progression, which provides new insight into the mechanisms underlying breast cancer metastasis and suggests a possible anticancer therapy for breast cancer.
中文翻译:

鞘磷脂合酶2通过破坏神经酰胺和鞘磷脂的同稳态而促进侵袭性乳腺癌表型。
乳腺癌是全世界女性中最常见的癌症类型,但尚不清楚肿瘤发生和发展的潜在机制。鞘磷脂合成酶2(SGMS2)是参与神经酰胺(Cer)和鞘磷脂(SM)同源转移的关键调节剂,目前主要研究其在脂质代谢中的作用。我们的主要研究表明,SGMS2高表达与乳腺癌转移有关。体外和体内功能获得和丧失功能测定表明,SGMS2通过抑制Cer相关途径的凋亡来促进癌细胞增殖,并通过增强TGF引起的上皮-间质转化(EMT)引发来促进癌细胞侵袭性。 -β/ Smad信号通路。进一步的研究确定SGMS2主要通过增加TGF-β1分泌来激活TGF-β/ Smad信号通路,这可能与SM的异常表达有关。因此,我们的发现表明,SGMS2介导的TGF-β/ Smad信号通路的激活在乳腺癌进展中很重要,这为乳腺癌转移的潜在机制提供了新的见识,并提出了可能的乳腺癌抗癌治疗方法。
更新日期:2019-02-15
中文翻译:

鞘磷脂合酶2通过破坏神经酰胺和鞘磷脂的同稳态而促进侵袭性乳腺癌表型。
乳腺癌是全世界女性中最常见的癌症类型,但尚不清楚肿瘤发生和发展的潜在机制。鞘磷脂合成酶2(SGMS2)是参与神经酰胺(Cer)和鞘磷脂(SM)同源转移的关键调节剂,目前主要研究其在脂质代谢中的作用。我们的主要研究表明,SGMS2高表达与乳腺癌转移有关。体外和体内功能获得和丧失功能测定表明,SGMS2通过抑制Cer相关途径的凋亡来促进癌细胞增殖,并通过增强TGF引起的上皮-间质转化(EMT)引发来促进癌细胞侵袭性。 -β/ Smad信号通路。进一步的研究确定SGMS2主要通过增加TGF-β1分泌来激活TGF-β/ Smad信号通路,这可能与SM的异常表达有关。因此,我们的发现表明,SGMS2介导的TGF-β/ Smad信号通路的激活在乳腺癌进展中很重要,这为乳腺癌转移的潜在机制提供了新的见识,并提出了可能的乳腺癌抗癌治疗方法。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号