当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
3-((R)-4-(((R)-6-(2-Bromo-4-fluorophenyl)-5-(ethoxycarbonyl)-2-(thiazol-2-yl)-3,6-dihydropyrimidin-4-yl)methyl)morpholin-2-yl)propanoic Acid (HEC72702), a Novel Hepatitis B Virus Capsid Inhibitor Based on Clinical Candidate GLS4
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-01-30 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01914 Qingyun Ren 1, 2 , Xinchang Liu 1, 2 , Guanghua Yan 1, 2 , Biao Nie 1 , Zhifu Zou 1, 2 , Jing Li 1 , Yunfu Chen 1 , Yu Wei 1 , Jianzhou Huang 1, 2 , Zhonghua Luo 1 , Baohua Gu 1 , Siegfried Goldmann 1, 2 , Jiancun Zhang 3 , Yingjun Zhang 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-01-30 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01914 Qingyun Ren 1, 2 , Xinchang Liu 1, 2 , Guanghua Yan 1, 2 , Biao Nie 1 , Zhifu Zou 1, 2 , Jing Li 1 , Yunfu Chen 1 , Yu Wei 1 , Jianzhou Huang 1, 2 , Zhonghua Luo 1 , Baohua Gu 1 , Siegfried Goldmann 1, 2 , Jiancun Zhang 3 , Yingjun Zhang 1
Affiliation
|
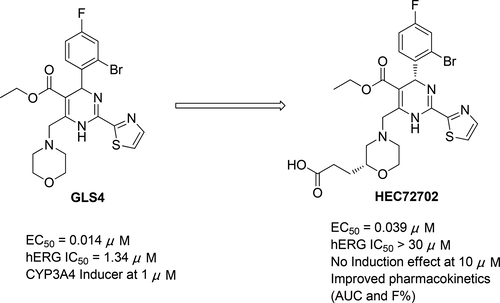
The inhibition of hepatitis B virus (HBV) capsid assembly is a novel strategy for the development of chronic hepatitis B (CHB) therapeutics. On the basis of the preclinical properties and clinical results of GLS4, we carried out further investigation to seek a better candidate compound with appropriate anti-HBV potency, reduced hERG activity, decreased CYP enzyme induction, and improved pharmacokinetic (PK) properties. To this end, we have successfully found that morpholine carboxyl analogues with comparable anti-HBV activities to that of GLS4 showed decreased hERG activities, but they displayed strong CYP3A4 induction in a concentration-dependent manner, except for morpholine propionic acid analogues. After several rounds of modification, compound 58 (HEC72702), which had an (R)-morpholine-2-propionic acid at the C6 position of its dihydropyrimidine core ring, was found to display no induction of the CYP1A2, CYP3A4, or CYP2B6 enzyme at the high concentration of 10 μM. In particular, it demonstrated a good systemic exposure and high oral bioavailability and achieved a viral-load reduction greater than 2 log in a hydrodynamic-injected (HDI) HBV mouse model and has now been selected for further development.
中文翻译:

3-((R)-4-(((R)-6-(2-溴-4-氟苯基)-5-(乙氧羰基)-2-(噻唑-2-基)-3,6-二氢嘧啶-4 -yl)甲基)吗啉-2-基)丙酸(HEC72702),一种基于临床候选GLS4的新型乙型肝炎病毒衣壳抑制剂
乙型肝炎病毒(HBV)衣壳装配的抑制是开发慢性乙型肝炎(CHB)治疗剂的一种新策略。根据GLS4的临床前特性和临床结果,我们进行了进一步的研究,以寻找具有适当的抗HBV效能,降低的hERG活性,降低的CYP酶诱导和改善的药代动力学(PK)特性的更好的候选化合物。为此,我们已经成功地发现,具有与GLS4相似的抗HBV活性的吗啉羧基类似物显示出降低的hERG活性,但是除了吗啉丙酸类似物外,它们还以浓度依赖的方式显示出强烈的CYP3A4诱导作用。经过几轮修改,化合物58(HEC72702),其中有一个(ř)在其二氢嘧啶核心环的C6位置的吗啉-2-丙酸被发现在10μM的高浓度下不显示CYP1A2,CYP3A4或CYP2B6酶的诱导作用。特别是,它表现出良好的全身暴露和较高的口服生物利用度,并且在水动力注射(HDI)HBV小鼠模型中实现了超过2 log的病毒载量降低,现已被选作进一步开发。
更新日期:2018-01-30
中文翻译:

3-((R)-4-(((R)-6-(2-溴-4-氟苯基)-5-(乙氧羰基)-2-(噻唑-2-基)-3,6-二氢嘧啶-4 -yl)甲基)吗啉-2-基)丙酸(HEC72702),一种基于临床候选GLS4的新型乙型肝炎病毒衣壳抑制剂
乙型肝炎病毒(HBV)衣壳装配的抑制是开发慢性乙型肝炎(CHB)治疗剂的一种新策略。根据GLS4的临床前特性和临床结果,我们进行了进一步的研究,以寻找具有适当的抗HBV效能,降低的hERG活性,降低的CYP酶诱导和改善的药代动力学(PK)特性的更好的候选化合物。为此,我们已经成功地发现,具有与GLS4相似的抗HBV活性的吗啉羧基类似物显示出降低的hERG活性,但是除了吗啉丙酸类似物外,它们还以浓度依赖的方式显示出强烈的CYP3A4诱导作用。经过几轮修改,化合物58(HEC72702),其中有一个(ř)在其二氢嘧啶核心环的C6位置的吗啉-2-丙酸被发现在10μM的高浓度下不显示CYP1A2,CYP3A4或CYP2B6酶的诱导作用。特别是,它表现出良好的全身暴露和较高的口服生物利用度,并且在水动力注射(HDI)HBV小鼠模型中实现了超过2 log的病毒载量降低,现已被选作进一步开发。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号