当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxidatively Induced Reductive Elimination: Exploring the Scope and Catalyst Systems with Ir, Rh, and Ru Complexes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-02-14 , DOI: 10.1021/jacs.9b00364
Jinwoo Kim 1, 2 , Kwangmin Shin 2 , Seongho Jin 1, 2 , Dongwook Kim 2 , Sukbok Chang 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-02-14 , DOI: 10.1021/jacs.9b00364
Jinwoo Kim 1, 2 , Kwangmin Shin 2 , Seongho Jin 1, 2 , Dongwook Kim 2 , Sukbok Chang 1, 2
Affiliation
|
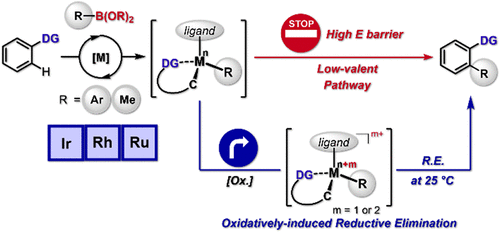
Direct conversion of C-H bonds into C-C bonds is a promising alternative to the conventional cross-coupling reactions, thus giving rise to a wide range of efficient catalytic C-H functionalization reactions. Among the elementary stages in the catalytic C-C bond formation, reductive elimination constitutes a key step of the catalytic cycle, and, therefore, extensive studies have been made to facilitate this process. In this regard, oxidation on the metal center of a post-transmetalation intermediate would be an appealing approach. Herein, we have explored the substrate scope, catalyst systems, and oxidation tools to prove that the oxidatively induced reductive elimination ( ORE) plays a critical role in the product-releasing C-C bond formation. Notably, we have demonstrated that ORE broadly operates with a series of half-sandwich d6 Ir(III)-, Rh(III)-, and Ru(II)-aryl complexes. We have described that the metal center oxidation of the isolable post-transmetalation intermediates by means of chemical- or electro-oxidation can readily deliver the desired arylated products upon reductive elimination even at ambient temperature. Computational studies delineated the thermodynamics of the reductive elimination, where the activation barriers are shown to be significantly reduced upon increasing the oxidation states of the intermediates. We were also successful in corroborating this ORE in the corresponding Rh- methyl complex. In addition, catalytic conditions were optimized to incorporate this mechanistic understanding into the Ir-, Rh-, and Ru-catalyzed C-C bond formations under mild conditions.
中文翻译:

氧化诱导的还原消除:探索 Ir、Rh 和 Ru 配合物的范围和催化剂系统
将 CH 键直接转化为 CC 键是传统交叉偶联反应的一种有前景的替代方法,从而产生了广泛的高效催化 CH 官能化反应。在催化 CC 键形成的基本阶段中,还原消除构成了催化循环的关键步骤,因此,已经进行了广泛的研究以促进这一过程。在这方面,金属转移后中间体金属中心的氧化将是一种有吸引力的方法。在此,我们探索了底物范围、催化剂系统和氧化工具,以证明氧化诱导还原消除 (ORE) 在释放产物的 CC 键形成中起着关键作用。值得注意的是,我们已经证明 ORE 广泛使用一系列半夹心 d6 Ir(III)-,Rh(III)-和Ru(II)-芳基配合物。我们已经描述了可分离的金属转移后中间体的金属中心氧化通过化学氧化或电氧化可以很容易地在还原消除后甚至在环境温度下提供所需的芳基化产物。计算研究描绘了还原消除的热力学,其中显示激活势垒随着中间体氧化态的增加而显着降低。我们还成功地在相应的 Rh-甲基复合物中证实了这种 ORE。此外,对催化条件进行了优化,以在温和条件下将这种机制理解纳入 Ir-、Rh- 和 Ru 催化的 CC 键形成中。我们已经描述了可分离的金属转移后中间体的金属中心氧化通过化学氧化或电氧化可以很容易地在还原消除后甚至在环境温度下提供所需的芳基化产物。计算研究描绘了还原消除的热力学,其中显示激活势垒随着中间体氧化态的增加而显着降低。我们还成功地在相应的 Rh-甲基复合物中证实了这种 ORE。此外,对催化条件进行了优化,以在温和条件下将这种机制理解纳入 Ir-、Rh- 和 Ru 催化的 CC 键形成中。我们已经描述了可分离的金属转移后中间体的金属中心氧化通过化学氧化或电氧化可以很容易地在还原消除后甚至在环境温度下提供所需的芳基化产物。计算研究描绘了还原消除的热力学,其中显示激活势垒随着中间体氧化态的增加而显着降低。我们还成功地在相应的 Rh-甲基复合物中证实了这种 ORE。此外,对催化条件进行了优化,以在温和条件下将这种机理理解纳入 Ir-、Rh- 和 Ru 催化的 CC 键形成中。
更新日期:2019-02-14
中文翻译:

氧化诱导的还原消除:探索 Ir、Rh 和 Ru 配合物的范围和催化剂系统
将 CH 键直接转化为 CC 键是传统交叉偶联反应的一种有前景的替代方法,从而产生了广泛的高效催化 CH 官能化反应。在催化 CC 键形成的基本阶段中,还原消除构成了催化循环的关键步骤,因此,已经进行了广泛的研究以促进这一过程。在这方面,金属转移后中间体金属中心的氧化将是一种有吸引力的方法。在此,我们探索了底物范围、催化剂系统和氧化工具,以证明氧化诱导还原消除 (ORE) 在释放产物的 CC 键形成中起着关键作用。值得注意的是,我们已经证明 ORE 广泛使用一系列半夹心 d6 Ir(III)-,Rh(III)-和Ru(II)-芳基配合物。我们已经描述了可分离的金属转移后中间体的金属中心氧化通过化学氧化或电氧化可以很容易地在还原消除后甚至在环境温度下提供所需的芳基化产物。计算研究描绘了还原消除的热力学,其中显示激活势垒随着中间体氧化态的增加而显着降低。我们还成功地在相应的 Rh-甲基复合物中证实了这种 ORE。此外,对催化条件进行了优化,以在温和条件下将这种机制理解纳入 Ir-、Rh- 和 Ru 催化的 CC 键形成中。我们已经描述了可分离的金属转移后中间体的金属中心氧化通过化学氧化或电氧化可以很容易地在还原消除后甚至在环境温度下提供所需的芳基化产物。计算研究描绘了还原消除的热力学,其中显示激活势垒随着中间体氧化态的增加而显着降低。我们还成功地在相应的 Rh-甲基复合物中证实了这种 ORE。此外,对催化条件进行了优化,以在温和条件下将这种机制理解纳入 Ir-、Rh- 和 Ru 催化的 CC 键形成中。我们已经描述了可分离的金属转移后中间体的金属中心氧化通过化学氧化或电氧化可以很容易地在还原消除后甚至在环境温度下提供所需的芳基化产物。计算研究描绘了还原消除的热力学,其中显示激活势垒随着中间体氧化态的增加而显着降低。我们还成功地在相应的 Rh-甲基复合物中证实了这种 ORE。此外,对催化条件进行了优化,以在温和条件下将这种机理理解纳入 Ir-、Rh- 和 Ru 催化的 CC 键形成中。

































 京公网安备 11010802027423号
京公网安备 11010802027423号