Nature Communications ( IF 14.7 ) Pub Date : 2019-02-14 , DOI: 10.1038/s41467-019-08669-1
Shengqing Zhu , Jian Qin , Fang Wang , Huan Li , Lingling Chu
|
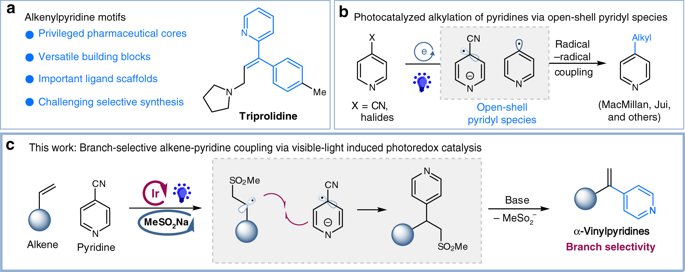
Alkenylpyridines are important pharmaceutical cores as well as versatile building blocks in organic synthesis. Heck reaction represents one of the most powerful platform for the construction of aryl-substituted alkenes, nevertheless, examples for Heck type coupling of alkenes with pyridines, particularly with branched selectivity, remain elusive. Here we report a catalytic, branch-selective pyridylation of alkenes via a sulfinate assisted photoredox catalysis. This reaction proceeds through a sequential radical addition/coupling/elimination, by utilizing readily available sodium sulfinates as reusable radical precursors as well as traceless elimination groups. This versatile protocol allows for the installation of important vinylpyridines with complete branched selectivity under mild conditions. Furthermore, this catalytic manifold is successfully applied to the expedient synthesis of Triprolidine.
中文翻译:

光氧化还原催化的烯烃的分支选择性吡啶基化,可方便地合成雷公藤rol
烯基吡啶是重要的药物核心,也是有机合成中的通用构建基块。Heck反应代表了用于构建芳基取代的烯烃的最强大的平台之一,但是,烯烃与吡啶的Heck型偶联,特别是具有分支选择性的Heck型偶联的例子仍然难以捉摸。在这里,我们报告通过亚磺酸盐辅助的光氧化还原催化的烯烃的催化,分支选择性吡啶化。通过利用容易获得的亚磺酸钠作为可重复使用的自由基前体以及无痕消除基团,该反应通过依次进行自由基加成/偶联/消除而进行。该通用协议允许在温和条件下以完全支化的选择性安装重要的乙烯基吡啶。此外,

































 京公网安备 11010802027423号
京公网安备 11010802027423号