当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tuning the Strength of the Resonance-Assisted Hydrogen Bond in o-Hydroxybenzaldehyde by Substitution in the Aromatic Ring1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-02-13 00:00:00 , DOI: 10.1021/acs.jpca.7b12066
Gerard Pareras 1 , Marcin Palusiak 2 , Miquel Duran 1 , Miquel Solà 1 , Sílvia Simon 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-02-13 00:00:00 , DOI: 10.1021/acs.jpca.7b12066
Gerard Pareras 1 , Marcin Palusiak 2 , Miquel Duran 1 , Miquel Solà 1 , Sílvia Simon 1
Affiliation
|
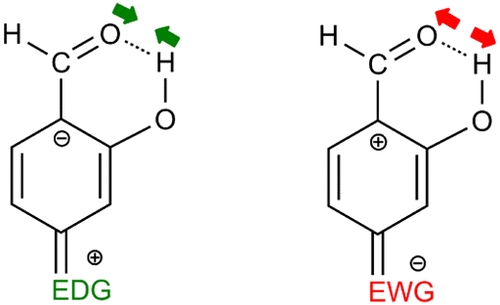
Intramolecular resonance-assisted hydrogen bonds (RAHBs) are stronger than conventional hydrogen bonds (HBs) thanks to the extra stabilization connected with the partial delocalization of the π-electrons within the HB motif containing conjugated formally single and double bonds. When these conjugated bonds are part of an aromatic ring, there is an interplay between resonance-assisted hydrogen bonding and the aromaticity of the ring. The main aim of the present work is to analyze the changes in RAHB strength by substitution in the aromatic ring. For this purpose, we use density functional theory methods to study all possible mono- and disubstitutions in the four free positions of the aromatic ring of o-hydroxybenzaldehyde. As substituents, we consider three π-electron donating groups (EDG: NH2, OH, and F) and three π-electron withdrawing groups (EWG: NO2, NO, and CN). We show that it is possible to tune the HB bond distance in the RAHB by locating different substituents in given positions of the aromatic ring. Indeed, certain combinations of EDG and EWD result in a reduction or increase of the HB distance by up to 0.05 Å. Results found can be explained by considering the existence of a resonance effect of the π-electrons within the HB motif.
更新日期:2018-02-13































 京公网安备 11010802027423号
京公网安备 11010802027423号