当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Succinylation Is a Gain-of-Function Modification in Human Lens αB-Crystallin
Biochemistry ( IF 2.9 ) Pub Date : 2019-02-13 00:00:00 , DOI: 10.1021/acs.biochem.8b01053 Sandip K. Nandi , Stefan Rakete , Rooban B. Nahomi , Cole Michel , Alexandra Dunbar , Kristofer S. Fritz , Ram H. Nagaraj
Biochemistry ( IF 2.9 ) Pub Date : 2019-02-13 00:00:00 , DOI: 10.1021/acs.biochem.8b01053 Sandip K. Nandi , Stefan Rakete , Rooban B. Nahomi , Cole Michel , Alexandra Dunbar , Kristofer S. Fritz , Ram H. Nagaraj
|
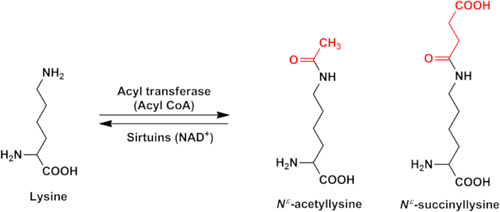
Acylation of lysine residues is a common post-translational modification of cellular proteins. Here, we show that lysine succinylation, a type of acylation, occurs in human lens proteins. All of the major crystallins exhibited Nε-succinyllysine (SuccK) residues. Quantification of SuccK in human lens proteins (from donors between the ages of 20 and 73 years) by LC–MS/MS showed a range between 1.2 and 14.3 pmol/mg lens protein. The total SuccK levels were slightly reduced in aged lenses (age > 60 years) relative to young lenses (age < 30 years). Immunohistochemical analyses revealed that SuccK was present in epithelium and fiber cells. Western blotting and immunoprecipitation experiments revealed that SuccK is particularly prominent in αB-crystallin, and succinylation in vitro revealed that αB-crystallin is more prone to succinylation than αA-crystallin. Mass spectrometric analyses showed succinylation at K72, K90, K92, K166, K175, and potentially K174 in human lens αB-crystallin. We detected succinylation at K72, K82, K90, K92, K103, K121, K150, K166, K175, and potentially K174 by mass spectrometry in mildly succinylated αB-crystallin. Mild succinylation improved the chaperone activity of αB-crystallin along with minor perturbation in tertiary and quaternary structure of the protein. These observations imply that succinylation is beneficial to αB-crystallin by improving its chaperone activity with only mild conformational alterations.
中文翻译:

琥珀酰化是人类晶状体αB-晶体中的功能增益修饰。
赖氨酸残基的酰化是细胞蛋白的常见翻译后修饰。在这里,我们显示出赖氨酸琥珀酰化(一种酰化作用)发生在人晶状体蛋白中。所有主要的晶状体的显示Ñ ε -succinyllysine(SuccK)残基。LC-MS / MS对人晶状体蛋白(来自年龄在20至73岁之间的供体)中SuccK的定量显示,晶状体蛋白的范围为1.2至14.3 pmol / mg。与年轻的镜片(年龄<30岁)相比,老年的镜片(年龄≥60岁)的总SuccK水平略有降低。免疫组织化学分析表明SuccK存在于上皮和纤维细胞中。Western印迹和免疫沉淀实验表明SuccK在αB-晶状体蛋白和体外琥珀酰化中尤为突出揭示αB-晶状体蛋白比αA-晶状体蛋白更易于琥珀酰化。质谱分析显示人晶状体αB-晶状体蛋白中K72,K90,K92,K166,K175和可能的K174处有琥珀酰化作用。我们通过质谱在轻度琥珀酰化的αB-晶状体蛋白中检测到K72,K82,K90,K92,K103,K121,K150,K166,K175和潜在的K174的琥珀酰化作用。轻度的琥珀酰化作用改善了蛋白的三级和四级结构的αB-晶状蛋白分子的伴侣活性以及较小的扰动。这些观察结果暗示,琥珀酰化仅通过轻微的构象改变就可以通过改善其伴侣活性而对αB-晶状体蛋白有益。
更新日期:2019-02-13
中文翻译:

琥珀酰化是人类晶状体αB-晶体中的功能增益修饰。
赖氨酸残基的酰化是细胞蛋白的常见翻译后修饰。在这里,我们显示出赖氨酸琥珀酰化(一种酰化作用)发生在人晶状体蛋白中。所有主要的晶状体的显示Ñ ε -succinyllysine(SuccK)残基。LC-MS / MS对人晶状体蛋白(来自年龄在20至73岁之间的供体)中SuccK的定量显示,晶状体蛋白的范围为1.2至14.3 pmol / mg。与年轻的镜片(年龄<30岁)相比,老年的镜片(年龄≥60岁)的总SuccK水平略有降低。免疫组织化学分析表明SuccK存在于上皮和纤维细胞中。Western印迹和免疫沉淀实验表明SuccK在αB-晶状体蛋白和体外琥珀酰化中尤为突出揭示αB-晶状体蛋白比αA-晶状体蛋白更易于琥珀酰化。质谱分析显示人晶状体αB-晶状体蛋白中K72,K90,K92,K166,K175和可能的K174处有琥珀酰化作用。我们通过质谱在轻度琥珀酰化的αB-晶状体蛋白中检测到K72,K82,K90,K92,K103,K121,K150,K166,K175和潜在的K174的琥珀酰化作用。轻度的琥珀酰化作用改善了蛋白的三级和四级结构的αB-晶状蛋白分子的伴侣活性以及较小的扰动。这些观察结果暗示,琥珀酰化仅通过轻微的构象改变就可以通过改善其伴侣活性而对αB-晶状体蛋白有益。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号