当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lewis Base/Copper Cooperatively Catalyzed Asymmetric α-Amination of Esters with Diaziridinones
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-01-29 , DOI: 10.1021/jacs.7b12628 Jin Song 1 , Zi-Jing Zhang 1 , Shu-Sen Chen 1 , Tao Fan 1 , Liu-Zhu Gong 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-01-29 , DOI: 10.1021/jacs.7b12628 Jin Song 1 , Zi-Jing Zhang 1 , Shu-Sen Chen 1 , Tao Fan 1 , Liu-Zhu Gong 1
Affiliation
|
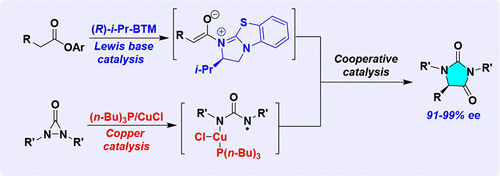
An enantioselective α-amination of esters by a Lewis base/copper(I) cooperative catalysis strategy has been developed. The transient chiral C1-ammonium enolate generated from pentafluorophenyl ester and nucleophilic Lewis base is nicely compatible with the copper intermediate formed from N, N-di- t-butyldiaziridinone and Cu(I) to allow for high levels of stereochemical control. The cooperative catalytic reaction leads to a diverse set of highly enantioenriched hydantoins in good yields with excellent enantioselectivities (90-99% ee).
中文翻译:

Lewis碱/铜协同催化酯与二氮丙啶酮的不对称α-胺化
已经开发了通过路易斯碱/铜(I)协同催化策略对酯进行对映选择性α-胺化。由五氟苯基酯和亲核路易斯碱生成的瞬态手性 C1-烯醇铵与由 N, N-二叔丁基二氮杂环丙烷酮和 Cu(I) 形成的铜中间体非常相容,以实现高水平的立体化学控制。协同催化反应以良好的收率和优异的对映选择性 (90-99% ee) 产生了多种高度对映体富集的乙内酰脲。
更新日期:2018-01-29
中文翻译:

Lewis碱/铜协同催化酯与二氮丙啶酮的不对称α-胺化
已经开发了通过路易斯碱/铜(I)协同催化策略对酯进行对映选择性α-胺化。由五氟苯基酯和亲核路易斯碱生成的瞬态手性 C1-烯醇铵与由 N, N-二叔丁基二氮杂环丙烷酮和 Cu(I) 形成的铜中间体非常相容,以实现高水平的立体化学控制。协同催化反应以良好的收率和优异的对映选择性 (90-99% ee) 产生了多种高度对映体富集的乙内酰脲。































 京公网安备 11010802027423号
京公网安备 11010802027423号