当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective One-Pot Synthesis of Biaryl-Substituted Amines by Combining Palladium and Enzyme Catalysis in Deep Eutectic Solvents
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2019-02-12 00:00:00 , DOI: 10.1021/acssuschemeng.8b06715
Juraj Paris 1, 2 , Aline Telzerow 3, 4 , Nicolás Ríos-Lombardía 1 , Kerstin Steiner 3 , Helmut Schwab 3 , Francisco Morís 1 , Harald Gröger 2 , Javier González-Sabín 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2019-02-12 00:00:00 , DOI: 10.1021/acssuschemeng.8b06715
Juraj Paris 1, 2 , Aline Telzerow 3, 4 , Nicolás Ríos-Lombardía 1 , Kerstin Steiner 3 , Helmut Schwab 3 , Francisco Morís 1 , Harald Gröger 2 , Javier González-Sabín 1
Affiliation
|
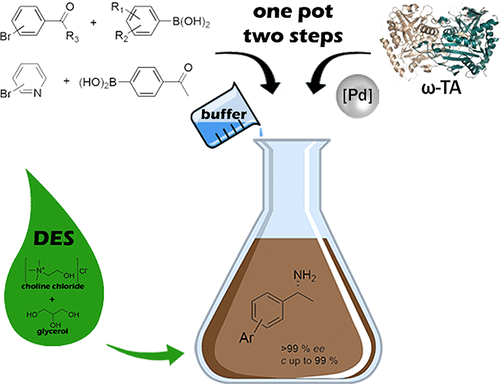
The first application of Deep Eutectic Solvents (DESs) in asymmetric bioamination of ketones has been accomplished. The amine transaminases (ATAs) turned out to be particularly stable in DES-buffer mixtures at a percentage of up to 75% (w/w) neoteric solvent. Moreover, this reaction medium was used to perform a chemoenzymatic cascade toward biaryl amines by coupling a Suzuki reaction sequentially with an enantioselective bioamination catalyzed by the recently discovered ATA from Exophiala xenobiotica (EX-ωTA). The solubilizing properties of DESs enabled the metal-catalyzed step at 200 mM loading of substrate and the subsequent biotransformation at 25 mM.
中文翻译:

深共晶溶剂中钯和酶催化的对映体一锅法合成联芳基取代的胺
的第一应用深共晶的溶剂(DESS)在酮的不对称bioamination已经完成。胺转氨酶(ATA)等被证明是在特别稳定的DES -buffer混合物在高达的百分比至75%(重量/重量)近代溶剂。此外,该反应介质被用于通过顺序地使铃木反应与由最近发现的来自异生菌的ATA (EX-ωTA)催化的对映选择性生物胺化偶合的Suzuki反应而朝联芳基胺进行化学酶联反应。DES的增溶特性使得在200 mM的底物负载下金属催化步骤成为可能,随后在25 mM的条件下进行了生物转化。
更新日期:2019-02-12
中文翻译:

深共晶溶剂中钯和酶催化的对映体一锅法合成联芳基取代的胺
的第一应用深共晶的溶剂(DESS)在酮的不对称bioamination已经完成。胺转氨酶(ATA)等被证明是在特别稳定的DES -buffer混合物在高达的百分比至75%(重量/重量)近代溶剂。此外,该反应介质被用于通过顺序地使铃木反应与由最近发现的来自异生菌的ATA (EX-ωTA)催化的对映选择性生物胺化偶合的Suzuki反应而朝联芳基胺进行化学酶联反应。DES的增溶特性使得在200 mM的底物负载下金属催化步骤成为可能,随后在25 mM的条件下进行了生物转化。































 京公网安备 11010802027423号
京公网安备 11010802027423号