当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Study on the regioselectivity of the N-ethylation reaction of N-benzyl-4-oxo-1,4-dihydroquinoline-3-carboxamide
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2019-02-12 , DOI: 10.3762/bjoc.15.35 Pedro N Batalha 1 , Luana da S M Forezi 1 , Maria Clara R Freitas 2, 3 , Nathalia M de C Tolentino 1 , Ednilsom Orestes 4 , José Walkimar de M Carneiro 1 , Fernanda da C S Boechat 1 , Maria Cecília B V de Souza 1
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2019-02-12 , DOI: 10.3762/bjoc.15.35 Pedro N Batalha 1 , Luana da S M Forezi 1 , Maria Clara R Freitas 2, 3 , Nathalia M de C Tolentino 1 , Ednilsom Orestes 4 , José Walkimar de M Carneiro 1 , Fernanda da C S Boechat 1 , Maria Cecília B V de Souza 1
Affiliation
|
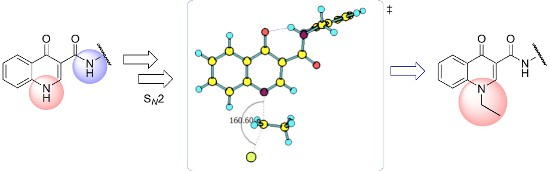
4-Oxoquinolines are a class of organic substances of great importance in medicinal chemistry, due to their biological and synthetic versatility. N-1-Alkylated-4-oxoquinoline derivatives have been associated with different pharmacological activities such as antibacterial and antiviral. The presence of a carboxamide unit connected to carbon C-3 of the 4-oxoquinoline core has been associated with various biological activities. Experimentally, the N-ethylation reaction of N-benzyl-4-oxo-1,4-dihydroquinoline-3-carboxamide occurs at the nitrogen of the oxoquinoline group, in a regiosselective way. In this work, we employed DFT methods to investigate the regiosselective ethylation reaction of N-benzyl-4-oxo-1,4-dihydroquinoline-3-carboxamide, evaluating its acid/base behavior and possible reaction paths.
中文翻译:

N-苄基-4-氧代-1,4-二氢喹啉-3-甲酰胺N-乙基化反应的区域选择性研究
4-氧代喹啉是一类在药物化学中非常重要的有机物质,因为它们具有生物和合成的多功能性。N -1-烷基化-4-氧代喹啉衍生物具有不同的药理活性,例如抗菌和抗病毒。与 4-氧代喹啉核心的 C-3 碳相连的甲酰胺单元的存在与多种生物活性有关。实验上, N-苄基-4-氧代-1,4-二氢喹啉-3-甲酰胺的N-乙基化反应以区域选择性方式发生在氧代喹啉基团的氮上。在这项工作中,我们采用DFT方法研究了N -benzyl-4-oxo-1,4-dihydroquinoline-3-carboxamide的区域选择性乙基化反应,评估其酸/碱行为和可能的反应路径。
更新日期:2019-02-13
中文翻译:

N-苄基-4-氧代-1,4-二氢喹啉-3-甲酰胺N-乙基化反应的区域选择性研究
4-氧代喹啉是一类在药物化学中非常重要的有机物质,因为它们具有生物和合成的多功能性。N -1-烷基化-4-氧代喹啉衍生物具有不同的药理活性,例如抗菌和抗病毒。与 4-氧代喹啉核心的 C-3 碳相连的甲酰胺单元的存在与多种生物活性有关。实验上, N-苄基-4-氧代-1,4-二氢喹啉-3-甲酰胺的N-乙基化反应以区域选择性方式发生在氧代喹啉基团的氮上。在这项工作中,我们采用DFT方法研究了N -benzyl-4-oxo-1,4-dihydroquinoline-3-carboxamide的区域选择性乙基化反应,评估其酸/碱行为和可能的反应路径。































 京公网安备 11010802027423号
京公网安备 11010802027423号