当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineering Atypical Tetracycline Formation in Amycolatopsis sulphurea for the Production of Modified Chelocardin Antibiotics.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2019-02-12 , DOI: 10.1021/acschembio.8b01125
Tadeja Lukežič 1, 2, 3 , Antoine Abou Fayad 1, 2 , Chantal Bader 1, 2 , Kirsten Harmrolfs 1, 2 , Johannes Bartuli 1, 2 , Sebastian Groß 1, 2 , Urška Lešnik 3, 4 , Fabienne Hennessen 1, 2 , Jennifer Herrmann 1, 2 , Špela Pikl 4 , Hrvoje Petković 4 , Rolf Müller 1, 2
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2019-02-12 , DOI: 10.1021/acschembio.8b01125
Tadeja Lukežič 1, 2, 3 , Antoine Abou Fayad 1, 2 , Chantal Bader 1, 2 , Kirsten Harmrolfs 1, 2 , Johannes Bartuli 1, 2 , Sebastian Groß 1, 2 , Urška Lešnik 3, 4 , Fabienne Hennessen 1, 2 , Jennifer Herrmann 1, 2 , Špela Pikl 4 , Hrvoje Petković 4 , Rolf Müller 1, 2
Affiliation
|
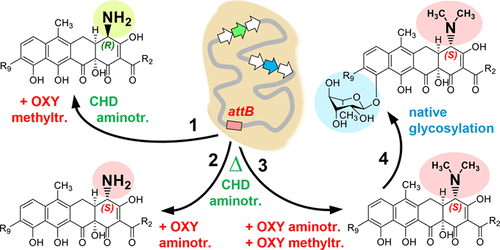
To combat the increasing spread of antimicrobial resistance and the shortage of novel anti-infectives, one strategy for the development of new antibiotics is to optimize known chemical scaffolds. Here, we focus on the biosynthetic engineering of Amycolatopsis sulphurea for derivatization of the atypical tetracycline chelocardin and its potent broad-spectrum derivative 2-carboxamido-2-deacetyl-chelocardin. Heterologous biosynthetic genes were introduced into this chelocardin producer to modify functional groups and generate new derivatives. We demonstrate cooperation of chelocardin polyketide synthase with tailoring enzymes involved in biosynthesis of oxytetracycline from Streptomyces rimosus. An interesting feature of chelocardin, compared with oxytetracycline, is the opposite stereochemistry of the C4 amino group. Genes involved in C4 transamination and N,N-dimethylation of oxytetracycline were heterologously expressed in an A. sulphurea mutant lacking C4-aminotransferase. Chelocardin derivatives with opposite stereochemistry of the C4 amino group, as N,N-dimethyl- epi-chelocardin and N,N-dimethyl-2-carboxamido-2-deacetyl- epi-chelocardin, were produced only when the aminotransferase from oxytetracycline was coexpressed with the N-methyltransferase OxyT. Surprisingly, OxyT exclusively accepted intermediates carrying an S-configured amino group at C4 in chelocardin. Applying medicinal chemistry approaches, several 2-carboxamido-2-deacetyl- epi-chelocardin derivatives modified at C4 were produced. Analysis of the antimicrobial activities of the modified compounds demonstrated that the primary amine in the R configuration is a crucial structural feature for activity of chelocardin. Unexpectedly, C10 glycosylated chelocardin analogues were identified, thus revealing the glycosylation potential of A. sulphurea. However, efficient glycosylation of the chelocardin backbone occurred only after engineering of a dimethylated amino group at the C4 position in the opposite S configuration, which suggests some evolutionary remains of chelocardin glycosylation.
中文翻译:

工程性非典型四环素形成在硫脲类支原体中,用于生产修饰的叶绿素类抗生素。
为了对抗日益增长的抗菌素耐药性和新型抗感染药的短缺,开发新抗生素的一种策略是优化已知的化学支架。在这里,我们专注于非典型四环素螯合素及其有效的广谱衍生物2-羧酰胺基-2-脱乙酰-螯合素的衍生化的扁桃硫脲的生物合成工程。异源生物合成基因被引入到该氯代人红素生产者中,以修饰官能团并产生新的衍生物。我们证明了螯合剂聚氯乙烯酮聚酮合酶与剪裁酶有关的合成酶从线状链霉菌的土霉素的生物合成中。与土霉素相比,氯氧卡丁素的一个有趣特征是C4氨基的立体化学相反。涉及土霉素的C4转氨和N,N-二甲基化的基因在缺少C4-氨基转移酶的硫脲曲霉突变体中异源表达。仅当共表达来自土霉素的氨基转移酶时,才产生具有与C4氨基相反的立体化学的螯合剂衍生物,例如N,N-二甲基-表-螯合剂和N,N-二甲基-2-羧酰胺基-2-脱乙酰基-表螯合剂。用N-甲基转移酶OxyT。令人惊讶的是,OxyT只接受了在氯氧卡丁素C4上带有S-构型氨基的中间体。应用药物化学方法,生产了几种在C4修饰的2-甲酰胺基-2-脱乙酰基-表螯合心苷衍生物。对改性化合物的抗菌活性的分析表明,R构型的伯胺是螯合心磷脂活性的关键结构特征。出乎意料的是,确定了C10糖基化的心卡素类似物,从而揭示了硫脲曲霉的糖基化潜力。然而,仅在以相反的S构型在C4位上的二甲基化氨基工程化之后,才发生了卡洛卡霉素主链的有效糖基化,这表明了卡洛卡丁糖基化的一些进化残留。
更新日期:2019-02-12
中文翻译:

工程性非典型四环素形成在硫脲类支原体中,用于生产修饰的叶绿素类抗生素。
为了对抗日益增长的抗菌素耐药性和新型抗感染药的短缺,开发新抗生素的一种策略是优化已知的化学支架。在这里,我们专注于非典型四环素螯合素及其有效的广谱衍生物2-羧酰胺基-2-脱乙酰-螯合素的衍生化的扁桃硫脲的生物合成工程。异源生物合成基因被引入到该氯代人红素生产者中,以修饰官能团并产生新的衍生物。我们证明了螯合剂聚氯乙烯酮聚酮合酶与剪裁酶有关的合成酶从线状链霉菌的土霉素的生物合成中。与土霉素相比,氯氧卡丁素的一个有趣特征是C4氨基的立体化学相反。涉及土霉素的C4转氨和N,N-二甲基化的基因在缺少C4-氨基转移酶的硫脲曲霉突变体中异源表达。仅当共表达来自土霉素的氨基转移酶时,才产生具有与C4氨基相反的立体化学的螯合剂衍生物,例如N,N-二甲基-表-螯合剂和N,N-二甲基-2-羧酰胺基-2-脱乙酰基-表螯合剂。用N-甲基转移酶OxyT。令人惊讶的是,OxyT只接受了在氯氧卡丁素C4上带有S-构型氨基的中间体。应用药物化学方法,生产了几种在C4修饰的2-甲酰胺基-2-脱乙酰基-表螯合心苷衍生物。对改性化合物的抗菌活性的分析表明,R构型的伯胺是螯合心磷脂活性的关键结构特征。出乎意料的是,确定了C10糖基化的心卡素类似物,从而揭示了硫脲曲霉的糖基化潜力。然而,仅在以相反的S构型在C4位上的二甲基化氨基工程化之后,才发生了卡洛卡霉素主链的有效糖基化,这表明了卡洛卡丁糖基化的一些进化残留。

































 京公网安备 11010802027423号
京公网安备 11010802027423号