Scientific Reports ( IF 3.8 ) Pub Date : 2019-02-11 , DOI: 10.1038/s41598-018-37866-z Thomas M Bocan 1, 2 , Falguni Basuli 3 , Robert G Stafford 1 , Jennifer L Brown 1, 4 , Xiang Zhang 3 , Allen J Duplantier 1, 2 , Rolf E Swenson 3
|
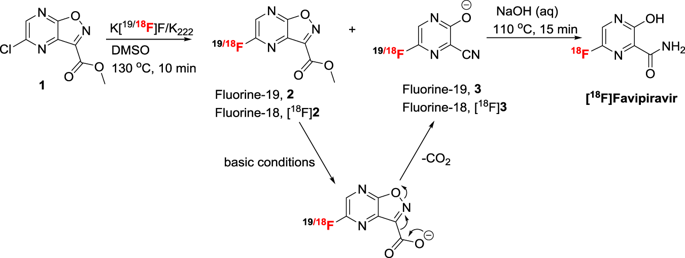
Favipiravir (T705; 6-fluoro-3-hydroxypyrazine-2-carboxamide) is a pyrazine analog that has demonstrated potent antiviral activity against a broad spectrum of viruses in multiple in vivo disease models. To better understand the compounds anti-viral activity, assessment of the drug’s biodistribution and kinetics in vivo may lend insight into how best to evaluate the compound efficacy preclinically and to contribute to the design of clinical studies to take into account the compound’s pharmacokinetic distribution and kinetics. In the current study, a method for synthesis of [18F]favipiravir was developed and the biodistribution in mice naïve to and pre-dosed with favipiravir was assessed by PET and gamma counting of tissue samples. Fluorine-18 labeling of favipiravir was achieved in a one-pot, two-step synthesis using a commercially available precursor, methyl-5-chloroisoxazolo[4,5-b]pyrazine-3-carboxylate, with an overall radiochemical yield of 15–24%, a molar activity of 37–74 GBq/µmol in a 70 minute synthesis time. [18F]favipiravir tissue uptake and distribution was similar in naïve and pre-dosed mice; however, in the pre-dosed animals plasma clearance was more rapid and tissue clearance appeared to be prolonged. In conclusion, application of PET to the evaluation of favipiravir has demonstrated the importance of dosing regimen on the distribution and tissue uptake and clearance of the molecule. Favipiravir is cleared through the kidney as previously reported but the liver and intestinal excretion may also play an important role in compound elimination. Measurement of the tissue uptake of favipiravir as determined by PET may be a more important indicator of a compound’s potential efficacy than purely monitoring plasma parameters such as viremia and drug levels.
中文翻译:

正电子发射断层扫描评估[18F]法维吡韦的合成及其在C3H / HeN小鼠中的生物分布。
Favipiravir(T705; 6-氟-3-羟基吡嗪-2-羧酰胺)是吡嗪类似物,已在多种体内疾病模型中显示出对多种病毒的有效抗病毒活性。为了更好地理解化合物的抗病毒活性,评估药物在体内的生物分布和动力学可能有助于深入了解如何最好地在临床前评估该化合物的功效,并有助于临床研究的设计,以考虑到该化合物的药代动力学分布和动力学。在目前的研究中,一种[ 18开发了F] favipiravir,并通过PET和组织样品的γ计数评估了未使用favipiravir并预用favipiravir的小鼠中的生物分布。使用市场上可买到的前体-5-氯异恶唑并[4,5-b]吡嗪-3-羧酸甲酯,通过一锅,两步合成法完成了favipiravir的氟-18标记,放射化学的总产率为15– 24%,在70分钟的合成时间内摩尔活性为37-74 GBq / µmol。[ 18F] favipiravir组织的摄取和分布在幼稚和预先给药的小鼠中相似;但是,在预先给药的动物中,血浆清除速度更快,并且组织清除似乎延长了。总之,将PET用于法维拉韦的评估已证明给药方案对分子的分布,组织摄取和清除的重要性。如前所述,法维吡韦可以通过肾脏清除,但是肝脏和肠道的排泄也可能在化合物清除中起重要作用。与单纯监测血浆参数(如病毒血症和药物水平)相比,通过PET测定的Favipiravir组织摄取的测量可能是化合物潜在功效的更重要指标。

































 京公网安备 11010802027423号
京公网安备 11010802027423号