当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of Mn2+, Ni2+, and Cu2+ on the Formation and Transformation of Hydrosulfate Green Rust: Reaction Processes and Underlying Mechanisms
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2019-02-08 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00187 Xiaoming Wang 1 , Jing Peng 1 , Xiaoliang Liang 2 , Mengqiang Zhu 3 , Bruno Lanson 4 , Lanxin Wang 1 , Xinran Liang 1 , Fan Liu 1 , Wenfeng Tan 1 , Xionghan Feng 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2019-02-08 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00187 Xiaoming Wang 1 , Jing Peng 1 , Xiaoliang Liang 2 , Mengqiang Zhu 3 , Bruno Lanson 4 , Lanxin Wang 1 , Xinran Liang 1 , Fan Liu 1 , Wenfeng Tan 1 , Xionghan Feng 1
Affiliation
|
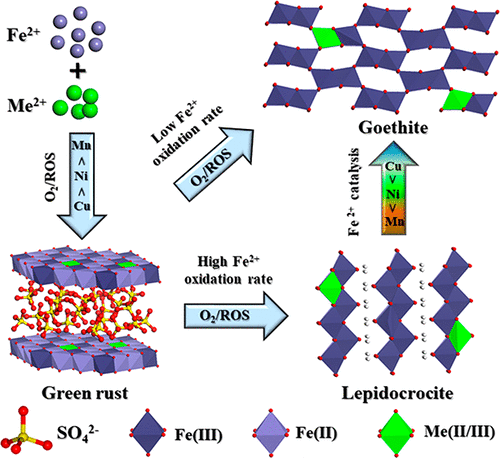
Green rusts (GRs), which are important intermediate phases during Fe2+ oxidation, are commonly associated with various metal cations during their crystallization in soils and sediments, but the effects of these foreign metal cations on the formation of GRs and on their subsequent transformation to Fe (hydr)oxides remain unclear. In the present study, the effects of Mn2+, Ni2+, and Cu2+ on the evolution processes of hydrosulfate green rust (GR2) are documented under various conditions and the mechanisms leading to cation incorporation in the reaction products are determined. The rates of GR2 formation and of its transformation to Fe (hydr)oxides both decrease in the order of Cu2+ > Ni2+ > Mn2+ and increase with increasing metal cation concentration. During GR2 crystallization, a small fraction of foreign metal cations is structurally incorporated in GR2 by replacing Fe2+, and their amount in the mineral follows the order of Cu2+ > Ni2+ > Mn2+. Under all conditions, the final reaction products are a mixture of lepidocrocite and goethite; a slow oxidation rate of mineral Fe2+ and a strong catalytic effect of surface Fe2+ both facilitate the goethite formation from GR2, reversely, favorable to lepidocrocite formation. Additionally, the three cations possess different speciation and distribution in lepidocrocite and goethite: Mn exists mainly as Mn(III) and probably minor Mn(II)–Mn(III) molecular clusters and occurs mainly in the mineral interior by isomorphic substitution or coated by the Fe (hydr)oxides crystals; Ni is present as Ni(II) and uniformly distributed in the newly formed minerals by either isomorphic substitution or surface adsorption; finally, Cu is mainly sorbed at the mineral surface as Cu(II) with minor Cu(I). These cations may thus be structurally incorporated in Fe oxides in the order of Mn(III) > Ni(II) > Cu(II). These new insights into the interaction between GR2 and trace metal cations improve our understanding of Fe oxide crystallization processes and of the environmental geochemical behavior of associated metal cations in redox alternating soils and sediments.
中文翻译:

Mn 2 +,Ni 2+和Cu 2+对硫酸氢盐绿锈形成和转化的影响:反应过程和机理
绿锈(GRs)是Fe 2+氧化过程中的重要中间阶段,通常在土壤和沉积物中结晶期间会与各种金属阳离子相关,但是这些外来金属阳离子对GRs的形成及其后续转化的影响氧化铁(氢)氧化物仍不清楚。在本研究中,记录了Mn 2 +,Ni 2+和Cu 2+在各种条件下对硫酸氢盐生铁锈(GR2)演变过程的影响,并确定了导致阳离子掺入反应产物的机理。GR2的形成速率及其向Fe(氢)氧化物的转化速率均按Cu 2+ > Ni 2+的顺序降低> Mn 2+并随着金属阳离子浓度的增加而增加。在GR2结晶过程中,一小部分外来金属阳离子通过置换Fe 2+在结构上掺入GR2 ,并且它们在矿物中的含量遵循Cu 2+ > Ni 2+ > Mn 2+的顺序。在所有条件下,最终的反应产物都是纤铁矿和针铁矿的混合物。矿物Fe 2+的缓慢氧化速率和表面Fe 2+的强催化作用两者都促进由GR2生成针铁矿,反之,则有利于纤铁云母的形成。另外,这三个阳离子在纤铁矿和针铁矿中具有不同的形态和分布:Mn主要以Mn(III)的形式存在,可能还存在少量的Mn(II)-Mn(III)分子簇,并且主要通过同构取代或被覆而存在于矿物内部。 Fe(氢)氧化物晶体;Ni以Ni(II)的形式存在,并通过同晶取代或表面吸附均匀地分布在新形成的矿物中。最后,铜主要以铜(II)和微量的铜(I)的形式吸附在矿物表面。这些阳离子因此可以以Mn(III)> Ni(II)> Cu(II)的顺序在结构上掺入Fe氧化物中。
更新日期:2019-02-08
中文翻译:

Mn 2 +,Ni 2+和Cu 2+对硫酸氢盐绿锈形成和转化的影响:反应过程和机理
绿锈(GRs)是Fe 2+氧化过程中的重要中间阶段,通常在土壤和沉积物中结晶期间会与各种金属阳离子相关,但是这些外来金属阳离子对GRs的形成及其后续转化的影响氧化铁(氢)氧化物仍不清楚。在本研究中,记录了Mn 2 +,Ni 2+和Cu 2+在各种条件下对硫酸氢盐生铁锈(GR2)演变过程的影响,并确定了导致阳离子掺入反应产物的机理。GR2的形成速率及其向Fe(氢)氧化物的转化速率均按Cu 2+ > Ni 2+的顺序降低> Mn 2+并随着金属阳离子浓度的增加而增加。在GR2结晶过程中,一小部分外来金属阳离子通过置换Fe 2+在结构上掺入GR2 ,并且它们在矿物中的含量遵循Cu 2+ > Ni 2+ > Mn 2+的顺序。在所有条件下,最终的反应产物都是纤铁矿和针铁矿的混合物。矿物Fe 2+的缓慢氧化速率和表面Fe 2+的强催化作用两者都促进由GR2生成针铁矿,反之,则有利于纤铁云母的形成。另外,这三个阳离子在纤铁矿和针铁矿中具有不同的形态和分布:Mn主要以Mn(III)的形式存在,可能还存在少量的Mn(II)-Mn(III)分子簇,并且主要通过同构取代或被覆而存在于矿物内部。 Fe(氢)氧化物晶体;Ni以Ni(II)的形式存在,并通过同晶取代或表面吸附均匀地分布在新形成的矿物中。最后,铜主要以铜(II)和微量的铜(I)的形式吸附在矿物表面。这些阳离子因此可以以Mn(III)> Ni(II)> Cu(II)的顺序在结构上掺入Fe氧化物中。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号