当前位置:
X-MOL 学术
›
Br. J. Cancer
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tumour cell blebbing and extracellular vesicle shedding: key role of matrikines and ribosomal protein SA.
British Journal of Cancer ( IF 6.4 ) Pub Date : 2019-02-11 , DOI: 10.1038/s41416-019-0382-0 Bertrand Brassart 1, 2 , Jordan Da Silva 1, 2 , Mélissa Donet 1, 2 , Emeline Seurat 1, 2 , Frédéric Hague 3 , Christine Terryn 4 , Fréderic Velard 5 , Jean Michel 6 , Halima Ouadid-Ahidouch 3 , Jean-Claude Monboisse 1, 2, 7 , Aleksander Hinek 8, 9, 10 , François-Xavier Maquart 1, 2, 7 , Laurent Ramont 1, 2, 7 , Sylvie Brassart-Pasco 1, 2
British Journal of Cancer ( IF 6.4 ) Pub Date : 2019-02-11 , DOI: 10.1038/s41416-019-0382-0 Bertrand Brassart 1, 2 , Jordan Da Silva 1, 2 , Mélissa Donet 1, 2 , Emeline Seurat 1, 2 , Frédéric Hague 3 , Christine Terryn 4 , Fréderic Velard 5 , Jean Michel 6 , Halima Ouadid-Ahidouch 3 , Jean-Claude Monboisse 1, 2, 7 , Aleksander Hinek 8, 9, 10 , François-Xavier Maquart 1, 2, 7 , Laurent Ramont 1, 2, 7 , Sylvie Brassart-Pasco 1, 2
Affiliation
|
BACKGROUND
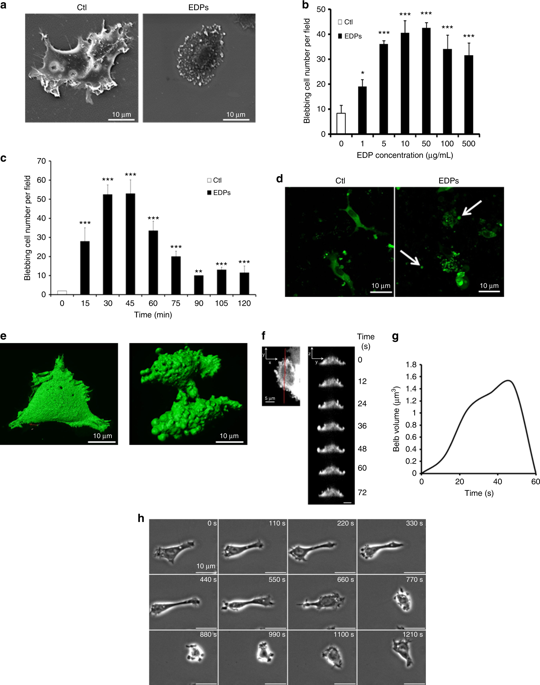
Carcinogenesis occurs in elastin-rich tissues and leads to local inflammation and elastolytic proteinase release. This contributes to bioactive matrix fragment (Matrikine) accumulation like elastin degradation products (EDP) stimulating tumour cell invasive and metastatic properties. We previously demonstrate that EDPs exert protumoural activities through Hsp90 secretion to stabilised extracellular proteinases.
METHODS
EDP influence on cancer cell blebbing and extracellular vesicle shedding were examined with a videomicroscope coupled with confocal Yokogawa spinning disk, by transmission electron microscopy, scanning electron microscopy and confocal microscopy. The ribosomal protein SA (RPSA) elastin receptor was identified after affinity chromatography by western blotting and cell immunolocalisation. mRNA expression was studied using real-time PCR. SiRNA were used to confirm the essential role of RPSA.
RESULTS
We demonstrate that extracellular matrix degradation products like EDPs induce tumour amoeboid phenotype with cell membrane blebbing and shedding of extracellular vesicle containing Hsp90 and proteinases in the extracellular space. EDPs influence intracellular calcium influx and cytoskeleton reorganisation. Among matrikines, VGVAPG and AGVPGLGVG peptides reproduced EDP effects through RPSA binding.
CONCLUSIONS
Our data suggests that matrikines induce cancer cell blebbing and extracellular vesicle release through RPSA binding, favouring dissemination, cell-to-cell communication and growth of cancer cells in metastatic sites.
中文翻译:

肿瘤细胞起泡和细胞外囊泡脱落:成熟蛋白和核糖体蛋白SA的关键作用。
背景技术致癌作用发生在富含弹性蛋白的组织中,并导致局部炎症和弹性蛋白酶的释放。这有助于像弹性蛋白降解产物(EDP)的生物活性基质片段(Matrikine)积累,从而刺激肿瘤细胞的侵袭和转移特性。我们先前证明,EDP通过Hsp90分泌发挥稳定的细胞外蛋白酶的促肿瘤活性。方法用电子显微镜结合共焦横河纺丝盘,通过透射电子显微镜,扫描电子显微镜和共聚焦显微镜检查EDP对癌细胞起泡和细胞外囊泡脱落的影响。核糖体蛋白SA(RPSA)弹性蛋白受体是在亲和层析后通过western blotting和细胞免疫定位鉴定的。使用实时PCR研究mRNA表达。SiRNA用于确认RPSA的重要作用。结果我们证明,诸如EDPs的细胞外基质降解产物可诱导肿瘤变形虫表型,细胞膜起泡并在细胞外空间中释放出含有Hsp90和蛋白酶的细胞外囊泡。EDP影响细胞内钙的内流和细胞骨架的重组。在matrikine中,VGVAPG和AGVPGLGVG肽通过RPSA结合重现了EDP的作用。结论我们的数据表明,基质激素通过RPSA结合诱导癌细胞起泡和细胞外囊泡释放,有利于癌细胞在转移部位的扩散,细胞间通讯和生长。结果我们证明,诸如EDPs的细胞外基质降解产物可诱导肿瘤变形虫表型,细胞膜起泡并在细胞外空间中释放出含有Hsp90和蛋白酶的细胞外囊泡。EDP影响细胞内钙的内流和细胞骨架的重组。在matrikine中,VGVAPG和AGVPGLGVG肽通过RPSA结合重现了EDP的作用。结论我们的数据表明,基质激素通过RPSA结合诱导癌细胞起泡和细胞外囊泡释放,有利于癌细胞在转移部位的扩散,细胞间通讯和生长。结果我们证明,诸如EDPs的细胞外基质降解产物可诱导肿瘤变形虫表型,细胞膜起泡并在细胞外空间中释放出含有Hsp90和蛋白酶的细胞外囊泡。EDP影响细胞内钙的内流和细胞骨架的重组。在matrikine中,VGVAPG和AGVPGLGVG肽通过RPSA结合重现了EDP的作用。结论我们的数据表明,基质激素通过RPSA结合诱导癌细胞起泡和细胞外囊泡释放,有利于癌细胞在转移部位的扩散,细胞间通讯和生长。EDP影响细胞内钙的内流和细胞骨架的重组。在matrikine中,VGVAPG和AGVPGLGVG肽通过RPSA结合重现了EDP的作用。结论我们的数据表明,基质激素通过RPSA结合诱导癌细胞起泡和细胞外囊泡释放,有利于癌细胞在转移部位的扩散,细胞间通讯和生长。EDP影响细胞内钙的内流和细胞骨架的重组。在matrikine中,VGVAPG和AGVPGLGVG肽通过RPSA结合重现了EDP的作用。结论我们的数据表明,基质激素通过RPSA结合诱导癌细胞起泡和细胞外囊泡释放,有利于癌细胞在转移部位的扩散,细胞间通讯和生长。
更新日期:2019-02-11
中文翻译:

肿瘤细胞起泡和细胞外囊泡脱落:成熟蛋白和核糖体蛋白SA的关键作用。
背景技术致癌作用发生在富含弹性蛋白的组织中,并导致局部炎症和弹性蛋白酶的释放。这有助于像弹性蛋白降解产物(EDP)的生物活性基质片段(Matrikine)积累,从而刺激肿瘤细胞的侵袭和转移特性。我们先前证明,EDP通过Hsp90分泌发挥稳定的细胞外蛋白酶的促肿瘤活性。方法用电子显微镜结合共焦横河纺丝盘,通过透射电子显微镜,扫描电子显微镜和共聚焦显微镜检查EDP对癌细胞起泡和细胞外囊泡脱落的影响。核糖体蛋白SA(RPSA)弹性蛋白受体是在亲和层析后通过western blotting和细胞免疫定位鉴定的。使用实时PCR研究mRNA表达。SiRNA用于确认RPSA的重要作用。结果我们证明,诸如EDPs的细胞外基质降解产物可诱导肿瘤变形虫表型,细胞膜起泡并在细胞外空间中释放出含有Hsp90和蛋白酶的细胞外囊泡。EDP影响细胞内钙的内流和细胞骨架的重组。在matrikine中,VGVAPG和AGVPGLGVG肽通过RPSA结合重现了EDP的作用。结论我们的数据表明,基质激素通过RPSA结合诱导癌细胞起泡和细胞外囊泡释放,有利于癌细胞在转移部位的扩散,细胞间通讯和生长。结果我们证明,诸如EDPs的细胞外基质降解产物可诱导肿瘤变形虫表型,细胞膜起泡并在细胞外空间中释放出含有Hsp90和蛋白酶的细胞外囊泡。EDP影响细胞内钙的内流和细胞骨架的重组。在matrikine中,VGVAPG和AGVPGLGVG肽通过RPSA结合重现了EDP的作用。结论我们的数据表明,基质激素通过RPSA结合诱导癌细胞起泡和细胞外囊泡释放,有利于癌细胞在转移部位的扩散,细胞间通讯和生长。结果我们证明,诸如EDPs的细胞外基质降解产物可诱导肿瘤变形虫表型,细胞膜起泡并在细胞外空间中释放出含有Hsp90和蛋白酶的细胞外囊泡。EDP影响细胞内钙的内流和细胞骨架的重组。在matrikine中,VGVAPG和AGVPGLGVG肽通过RPSA结合重现了EDP的作用。结论我们的数据表明,基质激素通过RPSA结合诱导癌细胞起泡和细胞外囊泡释放,有利于癌细胞在转移部位的扩散,细胞间通讯和生长。EDP影响细胞内钙的内流和细胞骨架的重组。在matrikine中,VGVAPG和AGVPGLGVG肽通过RPSA结合重现了EDP的作用。结论我们的数据表明,基质激素通过RPSA结合诱导癌细胞起泡和细胞外囊泡释放,有利于癌细胞在转移部位的扩散,细胞间通讯和生长。EDP影响细胞内钙的内流和细胞骨架的重组。在matrikine中,VGVAPG和AGVPGLGVG肽通过RPSA结合重现了EDP的作用。结论我们的数据表明,基质激素通过RPSA结合诱导癌细胞起泡和细胞外囊泡释放,有利于癌细胞在转移部位的扩散,细胞间通讯和生长。































 京公网安备 11010802027423号
京公网安备 11010802027423号