Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2019-02-10 , DOI: 10.1016/j.jmb.2019.02.001 Melanie H. Wong , Alexandra B. Samal , Mike Lee , Jiri Vlach , Nikolai Novikov , Anita Niedziela-Majka , Joy Y. Feng , Dmitry O. Koltun , Katherine M. Brendza , Hyock Joo Kwon , Brian E. Schultz , Roman Sakowicz , Jamil S. Saad , Giuseppe A. Papalia
|
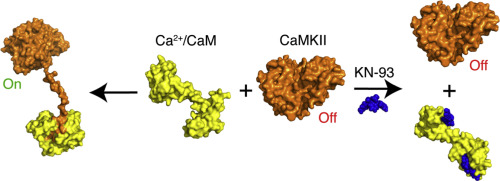
Calcium/calmodulin-dependent protein kinase II (CaMKII) is a multifunctional serine/threonine protein kinase that transmits calcium signals in various cellular processes. CaMKII is activated by calcium-bound calmodulin (Ca2+/CaM) through a direct binding mechanism involving a regulatory C-terminal α-helix in CaMKII. The Ca2+/CaM binding triggers transphosphorylation of critical threonine residues proximal to the CaM-binding site leading to the autoactivated state of CaMKII. The demonstration of its critical roles in pathophysiological processes has elevated CaMKII to a key target in the management of numerous diseases. The molecule KN-93 is the most widely used inhibitor for studying the cellular and in vivo functions of CaMKII. It is widely believed that KN-93 binds directly to CaMKII, thus preventing kinase activation by competing with Ca2+/CaM. Herein, we employed surface plasmon resonance, NMR, and isothermal titration calorimetry to characterize this presumed interaction. Our results revealed that KN-93 binds directly to Ca2+/CaM and not to CaMKII. This binding would disrupt the ability of Ca2+/CaM to interact with CaMKII, effectively inhibiting CaMKII activation. Our findings also indicated that KN-93 can specifically compete with a CaMKIIδ-derived peptide for binding to Ca2+/CaM. As indicated by the surface plasmon resonance and isothermal titration calorimetry data, apparently at least two KN-93 molecules can bind to Ca2+/CaM. Our findings provide new insight into how in vitro and in vivo data obtained with KN-93 should be interpreted. They further suggest that other Ca2+/CaM-dependent, non-CaMKII activities should be considered in KN-93–based mechanism-of-action studies and drug discovery efforts.
中文翻译:

KN-93分子通过与Ca 2+ / CaM结合来抑制钙/钙调蛋白依赖性蛋白激酶II(CaMKII)活性。
钙/钙调蛋白依赖性蛋白激酶II(CaMKII)是一种多功能的丝氨酸/苏氨酸蛋白激酶,可在各种细胞过程中传递钙信号。CaMKII被钙结合的钙调蛋白(Ca 2+ / CaM)通过涉及CaMKII中调节性C末端α-螺旋的直接结合机制激活。Ca 2+ / CaM结合触发接近CaM结合位点的关键苏氨酸残基的转磷酸化作用,从而导致CaMKII的自激活状态。CaMKII在病理生理过程中的关键作用已得到证明,已成为众多疾病管理中的关键目标。分子KN-93是研究细胞和体内最广泛使用的抑制剂CaMKII的功能。普遍认为,KN-93直接与CaMKII结合,从而通过与Ca 2+ / CaM竞争来阻止激酶活化。在这里,我们采用表面等离子体激元共振,NMR和等温滴定量热法来表征这种假定的相互作用。我们的结果表明,KN-93直接与Ca 2+ / CaM结合而不与CaMKII结合。这种结合会破坏Ca 2+ / CaM与CaMKII相互作用的能力,从而有效地抑制CaMKII的活化。我们的发现还表明,KN-93可以与CaMKIIδ衍生的肽竞争与Ca 2+ / CaM的结合。如表面等离振子共振和等温滴定热分析数据所示,显然至少有两个KN-93分子可以与Ca结合2+ / CaM。我们的发现为如何解释用KN-93获得的体外和体内数据提供了新的见解。他们进一步建议,在基于KN-93的作用机理研究和药物发现工作中,应考虑其他依赖Ca 2+ / CaM的非CaMKII活性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号