当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of sulfonamide, amide and amine hybrid pharmacophore, an entry of new class of carbonic anhydrase II inhibitors and evaluation of chemo-informatics and binding analysis.
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2019-02-10 , DOI: 10.1016/j.bioorg.2019.01.060 Attique Ahmed 1 , Pervaiz Ali Channar 1 , Aamer Saeed 1 , Markus Kalesse 2 , Mehar Ali Kazi 3 , Fayaz Ali Larik 1 , Qamar Abbas 4 , Mubashir Hassan 5 , Hussain Raza 5 , Sung-Yum Seo 5
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2019-02-10 , DOI: 10.1016/j.bioorg.2019.01.060 Attique Ahmed 1 , Pervaiz Ali Channar 1 , Aamer Saeed 1 , Markus Kalesse 2 , Mehar Ali Kazi 3 , Fayaz Ali Larik 1 , Qamar Abbas 4 , Mubashir Hassan 5 , Hussain Raza 5 , Sung-Yum Seo 5
Affiliation
|
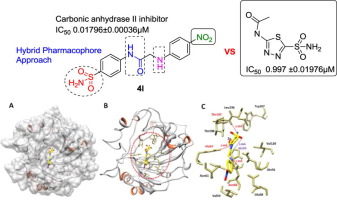
Selective inhibition of carbonic anhydrase (CA) enzyme is an active area of research for medicinal chemists. In the current account, a hybrid pharmacophore approach was employed to design sulfonamide, amide and amine containing new series of potent carbonic anhydrase II inhibitors. The aromatic fragment associated with pharmacophore was altered suitably in order to find effective inhibitors of CA-II. All the derivatives 4a-4m showed better inhibition compared to the standard acetazolamide. In particular, compound 4l exhibited significant inhibition with IC50 value of 0.01796 ± 0.00036 µM. The chemo-informatics analysis justified that all the designed compounds possess <10 HBA and <5 HBD. The ligands-protein binding analyses showed that 4l confined in the active binding pocket with three hydrogen bonds observed with His63, Asn66 and Thr197 residues.
中文翻译:

磺酰胺,酰胺和胺杂化药效团的合成,新型碳酸酐酶II抑制剂的进入以及化学信息学和结合分析的评估。
选择性抑制碳酸酐酶(CA)酶是药物化学家研究的一个活跃领域。在目前的帐户中,采用了一种混合药效团方法来设计磺酰胺,酰胺和胺类化合物,它们含有一系列新的有效的碳酸酐酶II抑制剂。为了找到有效的CA-II抑制剂,与药效团相关的芳族片段被适当地改变。与标准乙酰唑胺相比,所有衍生物4a-4m均显示出更好的抑制作用。特别地,化合物4l表现出显着的抑制,IC 50值为0.01796±0.00036μM。化学信息学分析证明所有设计的化合物均具有<10 HBA和<5 HBD。配体-蛋白质结合分析表明,His63观察到4l限制在活性结合袋中,带有三个氢键,
更新日期:2019-02-10
中文翻译:

磺酰胺,酰胺和胺杂化药效团的合成,新型碳酸酐酶II抑制剂的进入以及化学信息学和结合分析的评估。
选择性抑制碳酸酐酶(CA)酶是药物化学家研究的一个活跃领域。在目前的帐户中,采用了一种混合药效团方法来设计磺酰胺,酰胺和胺类化合物,它们含有一系列新的有效的碳酸酐酶II抑制剂。为了找到有效的CA-II抑制剂,与药效团相关的芳族片段被适当地改变。与标准乙酰唑胺相比,所有衍生物4a-4m均显示出更好的抑制作用。特别地,化合物4l表现出显着的抑制,IC 50值为0.01796±0.00036μM。化学信息学分析证明所有设计的化合物均具有<10 HBA和<5 HBD。配体-蛋白质结合分析表明,His63观察到4l限制在活性结合袋中,带有三个氢键,






























 京公网安备 11010802027423号
京公网安备 11010802027423号