Tetrahedron Letters ( IF 1.5 ) Pub Date : 2019-02-07 , DOI: 10.1016/j.tetlet.2019.02.013 Gabriela I. Matiello , Alessandra Pazini , Kácris I.M. da Silva , Rafaela G.M. da Costa , Günter Ebeling , Jairton Dupont , Jones Limberger , Jackson D. Scholten
|
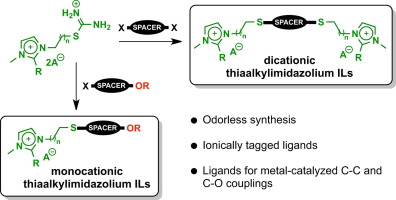
A simple and odorless route for the synthesis of monocationic and dicationic thiaalkylimidazolium ionic liquids (ILs) is reported. Our approach starts with the selective monoalkylation of dihalogenated substrates by methylimidazole derivatives, followed by the synthesis of odorless isothiouronium salts via reaction with thiourea. The target ILs are obtained after sequential hydrolysis-alkylation of the isothiouronium salts followed by anion metathesis in water. After extraction, the novel thiaalkylimidazolium ILs are obtained with high purity, without the requirement of additional purification steps. In order to demonstrate their applicability, two of these task-specific ILs were employed as ligands in Ullmann and Suzuki couplings and also as charged probes to detect copper intermediates via ESI(+)-MS.
中文翻译:

异硫脲盐用作合成噻烷基咪唑鎓离子液体的有用和无味的中间体
报道了一种简单且无味的路线,用于合成单阳离子和阳离子硫代烷基咪唑鎓离子液体(ILs)。我们的方法开始于通过甲基咪唑衍生物二卤代基板的选择性单烷基化,随后无味异硫脲盐的合成通过与硫脲反应。在异硫脲鎓盐连续水解烷基化,然后在水中进行阴离子复分解后,即可获得目标IL。提取后,无需额外的纯化步骤即可获得高纯度的新型硫代烷基咪唑鎓ILs。为了证明其适用性,这些任务特定的IL中的两个被用作Ullmann和Suzuki偶联的配体,还被用作带电探针以通过 ESI(+)-MS。































 京公网安备 11010802027423号
京公网安备 11010802027423号