当前位置:
X-MOL 学术
›
JAMA Intern. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of an In-Hospital Multifaceted Clinical Pharmacist Intervention on the Risk of Readmission
JAMA Internal Medicine ( IF 22.5 ) Pub Date : 2018-03-01 , DOI: 10.1001/jamainternmed.2017.8274 Lene Vestergaard Ravn-Nielsen 1 , Marie-Louise Duckert 1 , Mia Lolk Lund 1 , Jolene Pilegaard Henriksen 2 , Michelle Lyndgaard Nielsen 2 , Christina Skovsende Eriksen 3 , Thomas Croft Buck 4 , Anton Pottegård 1, 5 , Morten Rix Hansen 5, 6 , Jesper Hallas 5, 6
JAMA Internal Medicine ( IF 22.5 ) Pub Date : 2018-03-01 , DOI: 10.1001/jamainternmed.2017.8274 Lene Vestergaard Ravn-Nielsen 1 , Marie-Louise Duckert 1 , Mia Lolk Lund 1 , Jolene Pilegaard Henriksen 2 , Michelle Lyndgaard Nielsen 2 , Christina Skovsende Eriksen 3 , Thomas Croft Buck 4 , Anton Pottegård 1, 5 , Morten Rix Hansen 5, 6 , Jesper Hallas 5, 6
Affiliation
|
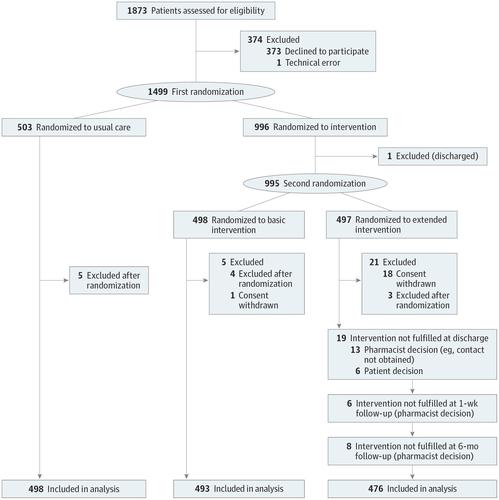
Importance Hospital readmissions are common among patients receiving multiple medications, with considerable costs to the patients and society. Objective To determine whether a multifaceted pharmacist intervention based on medication review, patient interview, and follow-up can reduce the number of readmissions and emergency department (ED) visits. Design, Setting, and Participants This randomized clinical multicenter study (Odense Pharmacist Trial Investigating Medication Interventions at Sector Transfer [OPTIMIST]) enrolled patients from September 1, 2013, through April 23, 2015, with a follow-up of 6 months completed on October 31, 2015. Consecutive medical patients in an acute admission ward who were 18 years or older and who used 5 or more medications were invited to participate. Of 1873 patients invited to participate, 1499 (80.0%) accepted. The medication review and patient interview were conducted in the hospital and followed up in collaboration with primary care. Analysis was based on intention to treat. Interventions The patients were randomized into 3 groups receiving usual care (no intervention), a basic intervention (medication review), and an extended intervention (medication review, 3 motivational interviews, and follow-up with the primary care physician, pharmacy, and nursing home). Main Outcomes and Measures The prespecified primary outcomes were readmission within 30 or 180 days and ED visits within 180 days. The primary composite end point was readmission or an ED visit within 180 days. Secondary outcomes were drug-related readmissions within 30 and 180 days after inclusion, and all-cause mortality and drug-related mortality. Results A total of 1467 patients (679 men [46.3%] and 788 women [53.7%]; median age, 72 years; interquartile range, 63-80 years) were part of the primary analysis, including 498 randomized to usual care, 493 randomized to the basic intervention, and 476 randomized to the extended intervention. The extended intervention had a significant effect on the numbers of patients who were readmitted within 30 days (hazard ratio [HR], 0.62; 95% CI, 0.46-0.84) or within 180 days (HR, 0.75; 95% CI, 0.62-0.90) after inclusion and on the number of patients who experienced the primary composite end point (HR, 0.77; 95% CI, 0.64-0.93). The study showed a nonsignificant reduction in drug-related readmissions within 30 days (HR, 0.65; 95% CI, 0.39-1.09) and within 180 days (HR, 0.80; 95% CI, 0.59-1.08) after inclusion and in deaths (HR, 0.83; 95% CI, 0.22-3.11). The number needed to treat to achieve the primary composite outcome for the extended intervention (vs usual care) was 12. Conclusions and Relevance A multifaceted clinical pharmacist intervention may reduce the number of ED visits and hospital readmissions. Trial Registration clinicaltrials.gov Identifier: NCT03079375
中文翻译:

院内多方面临床药师干预对再入院风险的影响
重要性 在接受多种药物治疗的患者中,再入院很常见,这给患者和社会带来了可观的成本。目的 确定基于药物审查、患者访谈和随访的多方面药剂师干预是否可以减少再入院和急诊科 (ED) 就诊的次数。设计、设置和参与者 这项随机临床多中心研究(Odense Pharmacist Trial Investigating Drug Interventions at Sector Transfer [OPTIMIST])从 2013 年 9 月 1 日至 2015 年 4 月 23 日招募了患者,并于 10 月完成了为期 6 个月的随访2015 年 3 月 31 日。邀请了 18 岁或以上且使用 5 种或更多药物的急性入院病房的连续内科患者参与。在受邀参加的 1873 名患者中,1499 名(80.0%)接受了。药物审查和患者访谈在医院进行,并与初级保健机构合作进行随访。分析基于意向治疗。干预 将患者随机分为 3 组,接受常规护理(无干预)、基本干预(药物审查)和扩展干预(药物审查、3 次动机访谈,以及对初级保健医师、药房和护理人员的随访)家)。主要结果和措施 预先指定的主要结果是 30 或 180 天内再入院和 180 天内就诊。主要复合终点是 180 天内再入院或 ED 就诊。次要结果是纳入后 30 和 180 天内与药物相关的再入院,以及全因死亡率和与药物相关的死亡率。结果 共有 1467 名患者(679 名男性 [46. 3%] 和 788 名女性 [53.7%];中位年龄,72 岁;四分位距(63-80 岁)是主要分析的一部分,其中 498 人随机接受常规治疗,493 人随机接受基本干预,476 人随机接受扩展干预。延长干预对 30 天内(风险比 [HR],0.62;95% CI,0.46-0.84)或 180 天内(HR,0.75;95% CI,0.62- 0.90)纳入后和经历主要复合终点的患者数量(HR,0.77;95% CI,0.64-0.93)。该研究显示,纳入后 30 天内(HR,0.65;95% CI,0.39-1.09)和 180 天内(HR,0.80;95% CI,0.59-1.08)和死亡人数无显着减少( HR,0.83;95% CI,0.22-3.11)。为实现扩展干预(相对于常规护理)的主要复合结果所需的治疗次数为 12。 结论和相关性 多方面的临床药师干预可能会减少 ED 就诊次数和再入院次数。试验注册clinicaltrials.gov 标识符:NCT03079375
更新日期:2018-03-01
中文翻译:

院内多方面临床药师干预对再入院风险的影响
重要性 在接受多种药物治疗的患者中,再入院很常见,这给患者和社会带来了可观的成本。目的 确定基于药物审查、患者访谈和随访的多方面药剂师干预是否可以减少再入院和急诊科 (ED) 就诊的次数。设计、设置和参与者 这项随机临床多中心研究(Odense Pharmacist Trial Investigating Drug Interventions at Sector Transfer [OPTIMIST])从 2013 年 9 月 1 日至 2015 年 4 月 23 日招募了患者,并于 10 月完成了为期 6 个月的随访2015 年 3 月 31 日。邀请了 18 岁或以上且使用 5 种或更多药物的急性入院病房的连续内科患者参与。在受邀参加的 1873 名患者中,1499 名(80.0%)接受了。药物审查和患者访谈在医院进行,并与初级保健机构合作进行随访。分析基于意向治疗。干预 将患者随机分为 3 组,接受常规护理(无干预)、基本干预(药物审查)和扩展干预(药物审查、3 次动机访谈,以及对初级保健医师、药房和护理人员的随访)家)。主要结果和措施 预先指定的主要结果是 30 或 180 天内再入院和 180 天内就诊。主要复合终点是 180 天内再入院或 ED 就诊。次要结果是纳入后 30 和 180 天内与药物相关的再入院,以及全因死亡率和与药物相关的死亡率。结果 共有 1467 名患者(679 名男性 [46. 3%] 和 788 名女性 [53.7%];中位年龄,72 岁;四分位距(63-80 岁)是主要分析的一部分,其中 498 人随机接受常规治疗,493 人随机接受基本干预,476 人随机接受扩展干预。延长干预对 30 天内(风险比 [HR],0.62;95% CI,0.46-0.84)或 180 天内(HR,0.75;95% CI,0.62- 0.90)纳入后和经历主要复合终点的患者数量(HR,0.77;95% CI,0.64-0.93)。该研究显示,纳入后 30 天内(HR,0.65;95% CI,0.39-1.09)和 180 天内(HR,0.80;95% CI,0.59-1.08)和死亡人数无显着减少( HR,0.83;95% CI,0.22-3.11)。为实现扩展干预(相对于常规护理)的主要复合结果所需的治疗次数为 12。 结论和相关性 多方面的临床药师干预可能会减少 ED 就诊次数和再入院次数。试验注册clinicaltrials.gov 标识符:NCT03079375































 京公网安备 11010802027423号
京公网安备 11010802027423号