Chemical Physics Letters ( IF 2.8 ) Pub Date : 2019-02-06 , DOI: 10.1016/j.cplett.2019.02.002 Shenghan Zhang , Yang Jiao
|
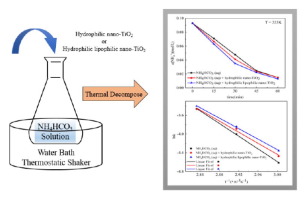
Aqueous ammonia is a potential absorbent for post-combustion carbon capture. Desorption of carbon dioxide (CO2) and ammonia (NH3) from ammonium bicarbonate (NH4HCO3) solution is one of key reactions of regeneration of the absorbent. Experimental studies on decomposition of NH4HCO3 solution with and without nano-TiO2 at different temperatures were conducted. Additions of nano-TiO2 into ammonium bicarbonate solutions decrease the apparent activation energy of overall decomposition reaction and accelerate the decomposition of ammonium bicarbonate solutions. Moreover, compared to hydrophilic nano-TiO2, hydrophilic lipophilic nano-TiO2 exerts more significant promoting effects on the decomposition reaction.
中文翻译:

亲水/亲脂纳米颗粒对NH 4 HCO 3溶液分解的影响
氨水是燃烧后碳捕获的潜在吸收剂。从碳酸氢铵(NH 4 HCO 3)溶液中解吸二氧化碳(CO 2)和氨(NH 3)是吸收剂再生的关键反应之一。进行了有和没有纳米TiO 2的NH 4 HCO 3溶液在不同温度下分解的实验研究。向碳酸氢铵溶液中添加纳米TiO 2降低了整体分解反应的表观活化能,并加速了碳酸氢铵溶液的分解。此外,与亲水性纳米TiO 2相比,亲脂性亲水性纳米TiO 2对分解反应具有更显着的促进作用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号