Nature Communications ( IF 14.7 ) Pub Date : 2019-02-07 , DOI: 10.1038/s41467-019-08419-3
Bingzhang Lu 1 , Lin Guo 1, 2 , Feng Wu 1 , Yi Peng 1 , Jia En Lu 1 , Tyler J Smart 3 , Nan Wang 4 , Y Zou Finfrock 5, 6 , David Morris 7 , Peng Zhang 7 , Ning Li 8, 9 , Peng Gao 8, 9, 10 , Yuan Ping 1 , Shaowei Chen 1, 4
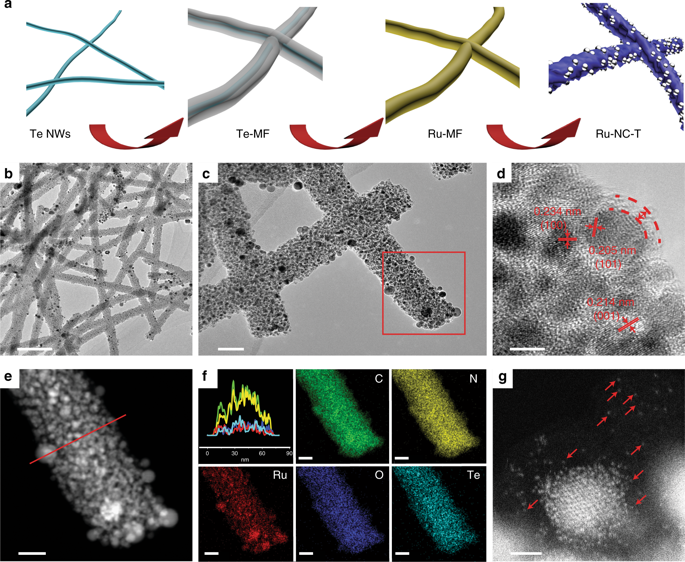
|
Hydrogen evolution reaction is an important process in electrochemical energy technologies. Herein, ruthenium and nitrogen codoped carbon nanowires are prepared as effective hydrogen evolution catalysts. The catalytic performance is markedly better than that of commercial platinum catalyst, with an overpotential of only −12 mV to reach the current density of 10 mV cm-2 in 1 M KOH and −47 mV in 0.1 M KOH. Comparisons with control experiments suggest that the remarkable activity is mainly ascribed to individual ruthenium atoms embedded within the carbon matrix, with minimal contributions from ruthenium nanoparticles. Consistent results are obtained in first-principles calculations, where RuCxNy moieties are found to show a much lower hydrogen binding energy than ruthenium nanoparticles, and a lower kinetic barrier for water dissociation than platinum. Among these, RuC2N2 stands out as the most active catalytic center, where both ruthenium and adjacent carbon atoms are the possible active sites.
中文翻译:

原子分散在碳中的钌在碱性介质中析氢方面优于铂
析氢反应是电化学能源技术中的一个重要过程。本文制备了钌和氮共掺杂碳纳米线作为有效的析氢催化剂。该催化性能明显优于商业铂催化剂,在1 M KOH中达到10 mV cm -2的电流密度的过电位仅为-12 mV,在0.1 M KOH中达到-47 mV的电流密度。与对照实验的比较表明,显着的活性主要归因于嵌入碳基质中的单个钌原子,钌纳米粒子的贡献最小。在第一性原理计算中获得了一致的结果,其中发现 RuC x N y部分显示出比钌纳米颗粒低得多的氢结合能,并且比铂具有更低的水解离动力学势垒。其中,RuC 2 N 2是最活跃的催化中心,其中钌和相邻的碳原子都是可能的活性位点。

































 京公网安备 11010802027423号
京公网安备 11010802027423号