Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The CH25H–CYP7B1–RORα axis of cholesterol metabolism regulates osteoarthritis
Nature ( IF 50.5 ) Pub Date : 2019-02-01 , DOI: 10.1038/s41586-019-0920-1 Wan-Su Choi 1 , Gyuseok Lee 1, 2 , Won-Hyun Song 2 , Jeong-Tae Koh 2 , Jiye Yang 1 , Ji-Sun Kwak 1 , Hyo-Eun Kim 1 , Seul Ki Kim 1 , Young-Ok Son 1 , Hojung Nam 3 , Iljung Jin 3 , Zee-Yong Park 4 , Jiyeon Kim 4 , In Young Park 5 , Jeong-Im Hong 5 , Hyun Ah Kim 5 , Churl-Hong Chun 6 , Je-Hwang Ryu 2 , Jang-Soo Chun 1
Nature ( IF 50.5 ) Pub Date : 2019-02-01 , DOI: 10.1038/s41586-019-0920-1 Wan-Su Choi 1 , Gyuseok Lee 1, 2 , Won-Hyun Song 2 , Jeong-Tae Koh 2 , Jiye Yang 1 , Ji-Sun Kwak 1 , Hyo-Eun Kim 1 , Seul Ki Kim 1 , Young-Ok Son 1 , Hojung Nam 3 , Iljung Jin 3 , Zee-Yong Park 4 , Jiyeon Kim 4 , In Young Park 5 , Jeong-Im Hong 5 , Hyun Ah Kim 5 , Churl-Hong Chun 6 , Je-Hwang Ryu 2 , Jang-Soo Chun 1
Affiliation
|
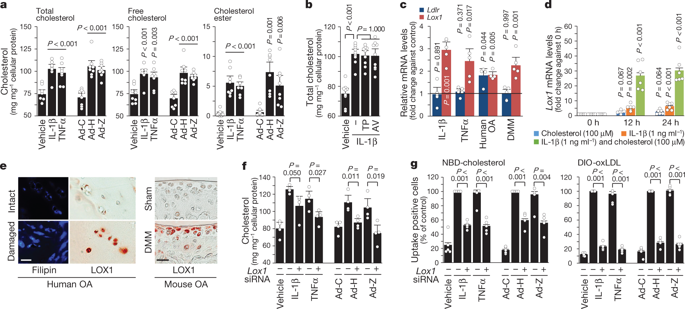
Osteoarthritis—the most common form of age-related degenerative whole-joint disease1—is primarily characterized by cartilage destruction, as well as by synovial inflammation, osteophyte formation and subchondral bone remodelling2,3. However, the molecular mechanisms that underlie the pathogenesis of osteoarthritis are largely unknown. Although osteoarthritis is currently considered to be associated with metabolic disorders, direct evidence for this is lacking, and the role of cholesterol metabolism in the pathogenesis of osteoarthritis has not been fully investigated4–6. Various types of cholesterol hydroxylases contribute to cholesterol metabolism in extrahepatic tissues by converting cellular cholesterol to circulating oxysterols, which regulate diverse biological processes7,8. Here we show that the CH25H–CYP7B1–RORα axis of cholesterol metabolism in chondrocytes is a crucial catabolic regulator of the pathogenesis of osteoarthritis. Osteoarthritic chondrocytes had increased levels of cholesterol because of enhanced uptake, upregulation of cholesterol hydroxylases (CH25H and CYP7B1) and increased production of oxysterol metabolites. Adenoviral overexpression of CH25H or CYP7B1 in mouse joint tissues caused experimental osteoarthritis, whereas knockout or knockdown of these hydroxylases abrogated the pathogenesis of osteoarthritis. Moreover, retinoic acid-related orphan receptor alpha (RORα) was found to mediate the induction of osteoarthritis by alterations in cholesterol metabolism. These results indicate that osteoarthritis is a disease associated with metabolic disorders and suggest that targeting the CH25H–CYP7B1–RORα axis of cholesterol metabolism may provide a therapeutic avenue for treating osteoarthritis.In experimentally induced osteoarthritis, chondrocytes show increased uptake and metabolism of cholesterol, implicating the CH25H–CYP7B1–RORα axis in the pathogenesis of osteoarthritis.
中文翻译:

胆固醇代谢的 CH25H-CYP7B1-RORα 轴调节骨关节炎
骨关节炎是与年龄相关的退行性全关节疾病的最常见形式 1,其主要特征是软骨破坏、滑膜炎症、骨赘形成和软骨下骨重塑 2、3。然而,骨关节炎发病机制的分子机制在很大程度上是未知的。尽管目前认为骨关节炎与代谢紊乱有关,但缺乏直接证据,胆固醇代谢在骨关节炎发病机制中的作用尚未得到充分研究4-6。各种类型的胆固醇羟化酶通过将细胞胆固醇转化为循环氧固醇来促进肝外组织中的胆固醇代谢,从而调节多种生物过程 7,8。在这里,我们表明软骨细胞中胆固醇代谢的 CH25H-CYP7B1-RORα 轴是骨关节炎发病机制的关键分解代谢调节剂。由于摄取增加、胆固醇羟化酶(CH25H 和 CYP7B1)的上调和氧固醇代谢物的产生增加,骨关节炎软骨细胞的胆固醇水平升高。CH25H 或 CYP7B1 在小鼠关节组织中的腺病毒过表达导致实验性骨关节炎,而这些羟化酶的敲除或敲低消除了骨关节炎的发病机制。此外,发现视黄酸相关孤儿受体α(RORα)通过胆固醇代谢的改变介导骨关节炎的诱导。
更新日期:2019-02-01
中文翻译:

胆固醇代谢的 CH25H-CYP7B1-RORα 轴调节骨关节炎
骨关节炎是与年龄相关的退行性全关节疾病的最常见形式 1,其主要特征是软骨破坏、滑膜炎症、骨赘形成和软骨下骨重塑 2、3。然而,骨关节炎发病机制的分子机制在很大程度上是未知的。尽管目前认为骨关节炎与代谢紊乱有关,但缺乏直接证据,胆固醇代谢在骨关节炎发病机制中的作用尚未得到充分研究4-6。各种类型的胆固醇羟化酶通过将细胞胆固醇转化为循环氧固醇来促进肝外组织中的胆固醇代谢,从而调节多种生物过程 7,8。在这里,我们表明软骨细胞中胆固醇代谢的 CH25H-CYP7B1-RORα 轴是骨关节炎发病机制的关键分解代谢调节剂。由于摄取增加、胆固醇羟化酶(CH25H 和 CYP7B1)的上调和氧固醇代谢物的产生增加,骨关节炎软骨细胞的胆固醇水平升高。CH25H 或 CYP7B1 在小鼠关节组织中的腺病毒过表达导致实验性骨关节炎,而这些羟化酶的敲除或敲低消除了骨关节炎的发病机制。此外,发现视黄酸相关孤儿受体α(RORα)通过胆固醇代谢的改变介导骨关节炎的诱导。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号