当前位置:
X-MOL 学术
›
Lancet Oncol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): a double-blind, randomised, placebo-controlled, phase 3 trial.
The Lancet Oncology ( IF 41.6 ) Pub Date : 2019-02-01 , DOI: 10.1016/s1470-2045(18)30791-5 Charles S Fuchs 1 , Kohei Shitara 2 , Maria Di Bartolomeo 3 , Sara Lonardi 4 , Salah-Eddin Al-Batran 5 , Eric Van Cutsem 6 , David H Ilson 7 , Maria Alsina 8 , Ian Chau 9 , Jill Lacy 1 , Michel Ducreux 10 , Guillermo Ariel Mendez 11 , Alejandro Molina Alavez 12 , Daisuke Takahari 13 , Wasat Mansoor 14 , Peter C Enzinger 15 , Vera Gorbounova 16 , Zev A Wainberg 17 , Susanna Hegewisch-Becker 18 , David Ferry 19 , Ji Lin 20 , Roberto Carlesi 21 , Mayukh Das 20 , Manish A Shah 22 ,
中文翻译:

Ramucirumab 联合顺铂和氟嘧啶作为转移性胃或交界腺癌患者的一线治疗 (RAINFALL):一项双盲、随机、安慰剂对照 3 期试验。
VEGF 和 VEGF 受体 2 (VEGFR-2) 介导的信号传导和血管生成可能有助于胃癌的发病机制和进展。我们的目的是评估在一线化疗中添加雷莫芦单抗(一种 VEGFR-2 拮抗剂单克隆抗体)是否可以改善转移性胃或胃食管交界腺癌患者的预后。
对于这项在 20 个国家的 126 个中心进行的双盲、随机、安慰剂对照 3 期试验,我们招募了 18 岁或以上患有转移性 HER2 阴性胃或胃食管交界腺癌的患者(东部肿瘤合作组) (ECOG) 体能状态为 0 或 1,器官功能充足。符合条件的患者被随机分配 (1:1),通过交互式网络响应系统接受顺铂(80 mg/m 2 ,第一天)加卡培他滨(1000 mg/m 2 ,每天两次,持续 14 天),每 21 天一次,并在第 1 天和第 8 天使用雷莫芦单抗 (8 mg/kg) 或安慰剂,每 21 天一次。无法服用卡培他滨的患者允许使用 5-氟尿嘧啶(第 1-5 天静脉输注 800 mg/m 2 )。主要终点是研究者评估的无进展生存期,根据前 508 名患者的治疗意向进行分析。我们对主要终点进行了敏感性分析,包括对 CT 扫描的集中审查。总生存期是关键的次要终点。这项研究的注册号为 。
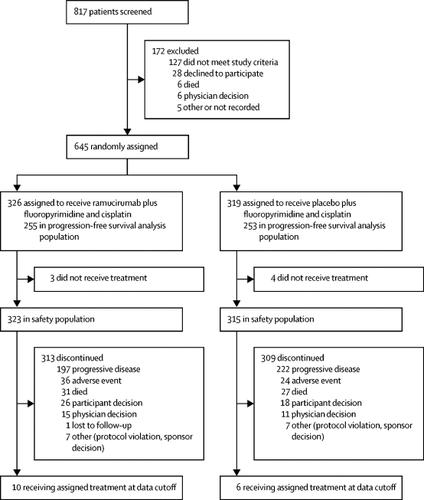
2015年1月28日至2016年9月16日期间,645名患者被随机分配接受雷莫芦单抗加氟嘧啶和顺铂治疗(n=326)或安慰剂加氟嘧啶和顺铂治疗(n=319)。研究人员评估的雷莫芦单抗组的无进展生存期显着长于安慰剂组(风险比 [HR] 0·753,95% CI 0·607–0·935,p=0·0106;中位无进展生存期) 5·7 个月 [5·5–6·5]与5·4 个月 [4·5–5·7])。基于放射学图像中央独立审查的敏感性分析并未证实研究者评估的无进展生存期差异(HR 0·961,95% CI 0·768–1·203,p=0·74)。各组之间的总生存期没有差异(0·962、0·801–1·156,p=0·6757;雷莫芦单抗组的中位总生存期为 11·2 个月 [9·9–11·9],而雷莫芦单抗组的中位总生存期为 10安慰剂组 7 个月 [9·5–11·9])。最常见的 3-4 级不良事件是中性粒细胞减少症(雷莫芦单抗组 323 例患者中有 85 例 [26%],安慰剂组 315 例患者中有85 例 [27%])、贫血(39 例 [12%]例 vs 44 例 [14%] ])和高血压(32 [10%] vs 5 [2%])。雷莫芦单抗组 323 名患者中,任何级别严重不良事件的发生率为 160 名(50%),安慰剂组 315 名患者中,任何级别严重不良事件的发生率为 149 名(47%)。最常见的严重不良事件是呕吐(雷莫芦单抗组为 14 例 [4%] ,安慰剂组为 21 例 [7%])和腹泻(雷莫芦单抗组为 11 例 [3%] 例,安慰剂组为19 例 [6%])。在研究治疗期间或停止研究治疗后 30 天内,每组均有 7 人死亡,这是治疗相关不良事件的结果。 在雷莫芦单抗组中,这些不良事件包括急性肾损伤、心脏骤停、胃出血、腹膜炎、气胸、感染性休克和猝死(各 n=1)。在安慰剂组中,这些不良事件包括脑血管意外(n=1)、多器官功能障碍综合征(n=2)、肺栓塞(n=2)、败血症(n=1)和小肠穿孔(n=1) )。
尽管无进展生存期的主要分析具有统计学意义,但这一结果并未在中央独立审查的无进展生存期敏感性分析中得到证实,并且没有改善总生存期。因此,不建议在顺铂加氟嘧啶化疗中添加雷莫芦单抗作为该患者群体的一线治疗。
更新日期:2019-03-02
The Lancet Oncology ( IF 41.6 ) Pub Date : 2019-02-01 , DOI: 10.1016/s1470-2045(18)30791-5 Charles S Fuchs 1 , Kohei Shitara 2 , Maria Di Bartolomeo 3 , Sara Lonardi 4 , Salah-Eddin Al-Batran 5 , Eric Van Cutsem 6 , David H Ilson 7 , Maria Alsina 8 , Ian Chau 9 , Jill Lacy 1 , Michel Ducreux 10 , Guillermo Ariel Mendez 11 , Alejandro Molina Alavez 12 , Daisuke Takahari 13 , Wasat Mansoor 14 , Peter C Enzinger 15 , Vera Gorbounova 16 , Zev A Wainberg 17 , Susanna Hegewisch-Becker 18 , David Ferry 19 , Ji Lin 20 , Roberto Carlesi 21 , Mayukh Das 20 , Manish A Shah 22 ,
Affiliation
|
Background
VEGF and VEGF receptor 2 (VEGFR-2)-mediated signalling and angiogenesis can contribute to the pathogenesis and progression of gastric cancer. We aimed to assess whether the addition of ramucirumab, a VEGFR-2 antagonist monoclonal antibody, to first-line chemotherapy improves outcomes in patients with metastatic gastric or gastro-oesophageal junction adenocarcinoma.Methods
For this double-blind, randomised, placebo-controlled, phase 3 trial done at 126 centres in 20 countries, we recruited patients aged 18 years or older with metastatic, HER2-negative gastric or gastro-oesophageal junction adenocarcinoma, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and adequate organ function. Eligible patients were randomly assigned (1:1) with an interactive web response system to receive cisplatin (80 mg/m 2, on the first day) plus capecitabine (1000 mg/m 2, twice daily for 14 days), every 21 days, and either ramucirumab (8 mg/kg) or placebo on days 1 and 8, every 21 days. 5-Fluorouracil (800 mg/m 2 intravenous infusion on days 1–5) was permitted in patients unable to take capecitabine. The primary endpoint was investigator-assessed progression-free survival, analysed by intention to treat in the first 508 patients. We did a sensitivity analysis of the primary endpoint, including a central review of CT scans. Overall survival was a key secondary endpoint. This study is registered with , number .Findings
Between Jan 28, 2015, and Sept 16, 2016, 645 patients were randomly assigned to receive ramucirumab plus fluoropyrimidine and cisplatin (n=326) or placebo plus fluoropyrimidine and cisplatin (n=319). Investigator-assessed progression-free survival was significantly longer in the ramucirumab group than the placebo group (hazard ratio [HR] 0·753, 95% CI 0·607–0·935, p=0·0106; median progression-free survival 5·7 months [5·5–6·5] vs 5·4 months [4·5–5·7]). A sensitivity analysis based on central independent review of the radiological images did not corroborate the investigator-assessed difference in progression-free survival (HR 0·961, 95% CI 0·768–1·203, p=0·74). There was no difference in overall survival between groups (0·962, 0·801–1·156, p=0·6757; median overall survival 11·2 months [9·9–11·9] in the ramucirumab group vs 10·7 months [9·5–11·9] in the placebo group). The most common grade 3–4 adverse events were neutropenia (85 [26%] of 323 patients in the ramucirumab group vs 85 [27%] of 315 in the placebo group), anaemia (39 [12%] vs 44 [14%]), and hypertension (32 [10%] vs 5 [2%]). The incidence of any-grade serious adverse events was 160 (50%) of 323 patients in the ramucirumab group and 149 (47%) of 315 patients in the placebo group. The most common serious adverse events were vomiting (14 [4%] in the ramucirumab group vs 21 [7%] in the placebo group) and diarrhoea (11 [3%] vs 19 [6%]). There were seven deaths in each group, either during study treatment or within 30 days of discontinuing study treatment, which were the result of treatment-related adverse events. In the ramucirumab group, these adverse events were acute kidney injury, cardiac arrest, gastric haemorrhage, peritonitis, pneumothorax, septic shock, and sudden death (n=1 of each). In the placebo group, these adverse events were cerebrovascular accident (n=1), multiple organ dysfunction syndrome (n=2), pulmonary embolism (n=2), sepsis (n=1), and small intestine perforation (n=1).Interpretation
Although the primary analysis for progression-free survival was statistically significant, this outcome was not confirmed in a sensitivity analysis of progression-free survival by central independent review, and did not improve overall survival. Therefore, the addition of ramucirumab to cisplatin plus fluoropyrimidine chemotherapy is not recommended as first-line treatment for this patient population.Funding
Eli Lilly and Company.中文翻译:

Ramucirumab 联合顺铂和氟嘧啶作为转移性胃或交界腺癌患者的一线治疗 (RAINFALL):一项双盲、随机、安慰剂对照 3 期试验。
背景
VEGF 和 VEGF 受体 2 (VEGFR-2) 介导的信号传导和血管生成可能有助于胃癌的发病机制和进展。我们的目的是评估在一线化疗中添加雷莫芦单抗(一种 VEGFR-2 拮抗剂单克隆抗体)是否可以改善转移性胃或胃食管交界腺癌患者的预后。
方法
对于这项在 20 个国家的 126 个中心进行的双盲、随机、安慰剂对照 3 期试验,我们招募了 18 岁或以上患有转移性 HER2 阴性胃或胃食管交界腺癌的患者(东部肿瘤合作组) (ECOG) 体能状态为 0 或 1,器官功能充足。符合条件的患者被随机分配 (1:1),通过交互式网络响应系统接受顺铂(80 mg/m 2 ,第一天)加卡培他滨(1000 mg/m 2 ,每天两次,持续 14 天),每 21 天一次,并在第 1 天和第 8 天使用雷莫芦单抗 (8 mg/kg) 或安慰剂,每 21 天一次。无法服用卡培他滨的患者允许使用 5-氟尿嘧啶(第 1-5 天静脉输注 800 mg/m 2 )。主要终点是研究者评估的无进展生存期,根据前 508 名患者的治疗意向进行分析。我们对主要终点进行了敏感性分析,包括对 CT 扫描的集中审查。总生存期是关键的次要终点。这项研究的注册号为 。
发现
2015年1月28日至2016年9月16日期间,645名患者被随机分配接受雷莫芦单抗加氟嘧啶和顺铂治疗(n=326)或安慰剂加氟嘧啶和顺铂治疗(n=319)。研究人员评估的雷莫芦单抗组的无进展生存期显着长于安慰剂组(风险比 [HR] 0·753,95% CI 0·607–0·935,p=0·0106;中位无进展生存期) 5·7 个月 [5·5–6·5]与5·4 个月 [4·5–5·7])。基于放射学图像中央独立审查的敏感性分析并未证实研究者评估的无进展生存期差异(HR 0·961,95% CI 0·768–1·203,p=0·74)。各组之间的总生存期没有差异(0·962、0·801–1·156,p=0·6757;雷莫芦单抗组的中位总生存期为 11·2 个月 [9·9–11·9],而雷莫芦单抗组的中位总生存期为 10安慰剂组 7 个月 [9·5–11·9])。最常见的 3-4 级不良事件是中性粒细胞减少症(雷莫芦单抗组 323 例患者中有 85 例 [26%],安慰剂组 315 例患者中有85 例 [27%])、贫血(39 例 [12%]例 vs 44 例 [14%] ])和高血压(32 [10%] vs 5 [2%])。雷莫芦单抗组 323 名患者中,任何级别严重不良事件的发生率为 160 名(50%),安慰剂组 315 名患者中,任何级别严重不良事件的发生率为 149 名(47%)。最常见的严重不良事件是呕吐(雷莫芦单抗组为 14 例 [4%] ,安慰剂组为 21 例 [7%])和腹泻(雷莫芦单抗组为 11 例 [3%] 例,安慰剂组为19 例 [6%])。在研究治疗期间或停止研究治疗后 30 天内,每组均有 7 人死亡,这是治疗相关不良事件的结果。 在雷莫芦单抗组中,这些不良事件包括急性肾损伤、心脏骤停、胃出血、腹膜炎、气胸、感染性休克和猝死(各 n=1)。在安慰剂组中,这些不良事件包括脑血管意外(n=1)、多器官功能障碍综合征(n=2)、肺栓塞(n=2)、败血症(n=1)和小肠穿孔(n=1) )。
解释
尽管无进展生存期的主要分析具有统计学意义,但这一结果并未在中央独立审查的无进展生存期敏感性分析中得到证实,并且没有改善总生存期。因此,不建议在顺铂加氟嘧啶化疗中添加雷莫芦单抗作为该患者群体的一线治疗。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号