当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formal [4 + 2] Cycloadditions of Anhydrides and α,β-Unsaturated N-Tosyl Ketimines
Organic Letters ( IF 4.9 ) Pub Date : 2019-02-05 00:00:00 , DOI: 10.1021/acs.orglett.8b04091 Noah P. Burlow 1 , Sara Y. Howard 1 , Carla M. Saunders 1 , James C. Fettinger 1 , Dean J. Tantillo 1 , Jared T. Shaw 1
Organic Letters ( IF 4.9 ) Pub Date : 2019-02-05 00:00:00 , DOI: 10.1021/acs.orglett.8b04091 Noah P. Burlow 1 , Sara Y. Howard 1 , Carla M. Saunders 1 , James C. Fettinger 1 , Dean J. Tantillo 1 , Jared T. Shaw 1
Affiliation
|
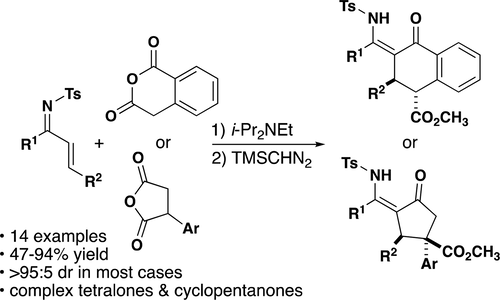
A method for the diastereoselective synthesis of highly substituted β-enamino ketones from anhydrides and ketone-derived imines is reported. Cyclic, enolizable anhydrides undergo a base-promoted conjugate addition reaction with α,β-unsaturated N-tosyl ketimines, followed by an intramolecular acylation to give formal [4 + 2] cycloaddition products. The carboxylic acid-containing products are formed with modest selectivity for the cis-diastereomer and can be fully epimerized to the trans-diastereomer upon esterification.
中文翻译:

酸酐与α,β-不饱和N-甲苯磺酰基酮亚胺的形式[4 + 2]的环加成反应
报道了一种由酸酐和酮衍生的亚胺非对映选择性合成高度取代的β-烯胺酮的方法。环状可烯醇化酸酐与α,β-不饱和N-甲苯磺酰基酮亚胺进行碱促进的共轭加成反应,然后进行分子内酰化,得到正式的[4 + 2]环加成产物。所形成的含羧酸的产物对顺式-非对映异构体具有适度的选择性,并且在酯化后可以完全差向异构为反式-非对映异构体。
更新日期:2019-02-05
中文翻译:

酸酐与α,β-不饱和N-甲苯磺酰基酮亚胺的形式[4 + 2]的环加成反应
报道了一种由酸酐和酮衍生的亚胺非对映选择性合成高度取代的β-烯胺酮的方法。环状可烯醇化酸酐与α,β-不饱和N-甲苯磺酰基酮亚胺进行碱促进的共轭加成反应,然后进行分子内酰化,得到正式的[4 + 2]环加成产物。所形成的含羧酸的产物对顺式-非对映异构体具有适度的选择性,并且在酯化后可以完全差向异构为反式-非对映异构体。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号