当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Novel Aryloxyethyl Derivatives of 1-(1-Benzoylpiperidin-4-yl)methanamine as the Extracellular Regulated Kinases 1/2 (ERK1/2) Phosphorylation-Preferring Serotonin 5-HT1A Receptor-Biased Agonists with Robust Antidepressant-like Activity.
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2019-03-02 , DOI: 10.1021/acs.jmedchem.9b00062 Joanna Sniecikowska 1 , Monika Gluch-Lutwin 1 , Adam Bucki 1 , Anna Więckowska 1 , Agata Siwek 1 , Magdalena Jastrzebska-Wiesek 1 , Anna Partyka 1 , Daria Wilczyńska 1 , Karolina Pytka 1 , Krzysztof Pociecha 1 , Agnieszka Cios 1 , Elżbieta Wyska 1 , Anna Wesołowska 1 , Maciej Pawłowski 1 , Mark A Varney 2 , Adrian Newman-Tancredi 2 , Marcin Kolaczkowski 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2019-03-02 , DOI: 10.1021/acs.jmedchem.9b00062 Joanna Sniecikowska 1 , Monika Gluch-Lutwin 1 , Adam Bucki 1 , Anna Więckowska 1 , Agata Siwek 1 , Magdalena Jastrzebska-Wiesek 1 , Anna Partyka 1 , Daria Wilczyńska 1 , Karolina Pytka 1 , Krzysztof Pociecha 1 , Agnieszka Cios 1 , Elżbieta Wyska 1 , Anna Wesołowska 1 , Maciej Pawłowski 1 , Mark A Varney 2 , Adrian Newman-Tancredi 2 , Marcin Kolaczkowski 1
Affiliation
|
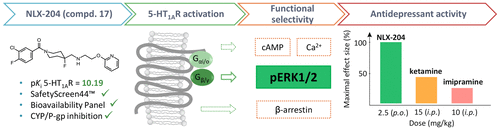
Novel 1-(1-benzoylpiperidin-4-yl)methanamine derivatives were designed as "biased agonists" of serotonin 5-HT1A receptors. The compounds were tested in signal transduction assays (ERK1/2 phosphorylation, cAMP inhibition, Ca2+ mobilization, and β-arrestin recruitment) which identified ERK1/2 phosphorylation-preferring aryloxyethyl derivatives. The novel series showed high 5-HT1A receptor affinity, >1000-fold selectivity versus noradrenergic α1, dopamine D2, serotonin 5-HT2A, histamine H1, and muscarinic M1 receptors, and favorable druglike properties (CNS-MPO, Fsp3, LELP). The lead structure, (3-chloro-4-fluorophenyl)(4-fluoro-4-(((2-(pyridin-2-yloxy)ethyl)amino)methyl)piperidin-1-yl)methanone (17, NLX-204), displayed high selectivity in the SafetyScreen44 panel (including hERG channel), high solubility, metabolic stability, and Caco-2 penetration and did not block CYP3A4, CYP2D6 isoenzymes, or P-glycoprotein. Preliminary in vivo studies confirmed its promising pharmacokinetic profile. 17 also robustly stimulated ERK1/2 phosphorylation in rat cortex and showed highly potent (MED = 0.16 mg/kg) and efficacious antidepressant-like activity, totally eliminating immobility in the rat Porsolt test. These data suggest that the present 5-HT1A receptor-biased agonists could constitute promising antidepressant drug candidates.
中文翻译:

1-(1-苯甲酰基哌啶丁-4-基)甲胺的新型芳氧基乙基衍生物作为细胞外调节激酶1/2(ERK1 / 2)磷酸化的首选5-羟色胺5-HT1A受体-激动剂,具有强大的抗抑郁样活性。
新型的1-(1-苯甲酰基哌啶-4-基)甲胺衍生物被设计为5-羟色胺5-HT1A受体的“偏向激动剂”。在信号转导分析(ERK1 / 2磷酸化,cAMP抑制,Ca2 +动员和β-arrestin募集)中测试了这些化合物,这些化合物鉴定出了ERK1 / 2磷酸化优先的芳氧基乙基衍生物。该新系列药物显示出较高的5-HT1A受体亲和力,相对于去甲肾上腺素α1,多巴胺D2、5-羟色胺5-HT2A,组胺H1和毒蕈碱M1受体具有> 1000倍的选择性,并具有良好的类药物特性(CNS-MPO,Fsp3,LELP)。铅结构(3-氯-4-氟苯基)(4-氟-4-((((2-(吡啶-2-基氧基)乙基)氨基)甲基)哌啶-1-基)甲酮(17,NLX- 204),在SafetyScreen44面板(包括hERG通道)中显示出高选择性,高溶解度,代谢稳定性,和Caco-2渗透,并且不阻断CYP3A4,CYP2D6同工酶或P-糖蛋白。初步的体内研究证实了其有希望的药代动力学特征。17还强烈刺激了大鼠皮层中的ERK1 / 2磷酸化,并显示出强效(MED = 0.16 mg / kg)和有效的抗抑郁剂样活性,完全消除了大鼠Porsolt试验中的固定性。这些数据表明,目前的5-HT1A受体偏向激动剂可以构成有希望的抗抑郁药候选物。完全消除了大鼠Porsolt测试中的固定性。这些数据表明,目前的5-HT1A受体偏向激动剂可以构成有希望的抗抑郁药候选物。完全消除了大鼠Porsolt测试中的固定性。这些数据表明,目前的5-HT1A受体偏向激动剂可以构成有希望的抗抑郁药候选物。
更新日期:2019-02-05
中文翻译:

1-(1-苯甲酰基哌啶丁-4-基)甲胺的新型芳氧基乙基衍生物作为细胞外调节激酶1/2(ERK1 / 2)磷酸化的首选5-羟色胺5-HT1A受体-激动剂,具有强大的抗抑郁样活性。
新型的1-(1-苯甲酰基哌啶-4-基)甲胺衍生物被设计为5-羟色胺5-HT1A受体的“偏向激动剂”。在信号转导分析(ERK1 / 2磷酸化,cAMP抑制,Ca2 +动员和β-arrestin募集)中测试了这些化合物,这些化合物鉴定出了ERK1 / 2磷酸化优先的芳氧基乙基衍生物。该新系列药物显示出较高的5-HT1A受体亲和力,相对于去甲肾上腺素α1,多巴胺D2、5-羟色胺5-HT2A,组胺H1和毒蕈碱M1受体具有> 1000倍的选择性,并具有良好的类药物特性(CNS-MPO,Fsp3,LELP)。铅结构(3-氯-4-氟苯基)(4-氟-4-((((2-(吡啶-2-基氧基)乙基)氨基)甲基)哌啶-1-基)甲酮(17,NLX- 204),在SafetyScreen44面板(包括hERG通道)中显示出高选择性,高溶解度,代谢稳定性,和Caco-2渗透,并且不阻断CYP3A4,CYP2D6同工酶或P-糖蛋白。初步的体内研究证实了其有希望的药代动力学特征。17还强烈刺激了大鼠皮层中的ERK1 / 2磷酸化,并显示出强效(MED = 0.16 mg / kg)和有效的抗抑郁剂样活性,完全消除了大鼠Porsolt试验中的固定性。这些数据表明,目前的5-HT1A受体偏向激动剂可以构成有希望的抗抑郁药候选物。完全消除了大鼠Porsolt测试中的固定性。这些数据表明,目前的5-HT1A受体偏向激动剂可以构成有希望的抗抑郁药候选物。完全消除了大鼠Porsolt测试中的固定性。这些数据表明,目前的5-HT1A受体偏向激动剂可以构成有希望的抗抑郁药候选物。








































 京公网安备 11010802027423号
京公网安备 11010802027423号