当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Substituent Effect in the Synthesis of α,α‐Dibromoketones, 1,2‐Dibromalkenes, and 1,2‐Diketones from the Reaction of Alkynes and Dibromoisocyanuric Acid
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2019-02-28 , DOI: 10.1002/adsc.201801535 Eunjeong Cho 1 , Aravindan Jayaraman 1 , Junseong Lee 1 , Kyoung Chul Ko 2 , Sunwoo Lee 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2019-02-28 , DOI: 10.1002/adsc.201801535 Eunjeong Cho 1 , Aravindan Jayaraman 1 , Junseong Lee 1 , Kyoung Chul Ko 2 , Sunwoo Lee 1
Affiliation
|
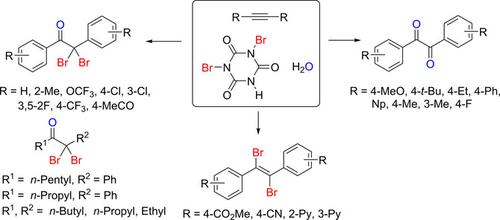
Internal alkynes reacted with dibromoisocyanuric acid/H2O to afford α,α‐dibromoketone and 1,2‐diketone derivatives. Diarylalkynes with activating groups provided 1,2‐diketone derivatives as the major products, whereas diarylalkynes with a non‐activating group or alkylarylalkynes gave α,α‐dibromoketone derivatives as the major products. In addition, diarylalkynes with deactivating groups provided 1,2‐dibromoalkenes. The reaction was conducted at room temperature and showed good yields in most cases. Reaction pathways have been proposed on the basis of experimental observations and density functional theory (DFT) calculations.
中文翻译:

炔烃与二溴异氰尿酸反应合成α,α-二溴酮,1,2-二溴代烯酮和1,2-二酮的替代作用
内部炔烃与二溴异氰尿酸/ H 2 O反应,得到α,α-二溴酮和1,2-二酮衍生物。具有活化基团的二芳基炔烃提供1,2-二酮衍生物作为主要产物,而具有非活化基团的二芳基炔烃或烷基芳基炔烃提供的α,α-二溴酮衍生物为主要产物。此外,带有失活基团的二芳基炔烃可提供1,2-二溴烯烃。该反应在室温下进行,并且在大多数情况下显示出良好的产率。已经基于实验观察和密度泛函理论(DFT)计算提出了反应途径。
更新日期:2019-02-28
中文翻译:

炔烃与二溴异氰尿酸反应合成α,α-二溴酮,1,2-二溴代烯酮和1,2-二酮的替代作用
内部炔烃与二溴异氰尿酸/ H 2 O反应,得到α,α-二溴酮和1,2-二酮衍生物。具有活化基团的二芳基炔烃提供1,2-二酮衍生物作为主要产物,而具有非活化基团的二芳基炔烃或烷基芳基炔烃提供的α,α-二溴酮衍生物为主要产物。此外,带有失活基团的二芳基炔烃可提供1,2-二溴烯烃。该反应在室温下进行,并且在大多数情况下显示出良好的产率。已经基于实验观察和密度泛函理论(DFT)计算提出了反应途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号