当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The mechanism of manganese dissolution on Li1.6Mn1.6O4 ion sieves with HCl†
Dalton Transactions ( IF 3.5 ) Pub Date : 2018-01-29 00:00:00 , DOI: 10.1039/c8dt00033f Aolei Gao 1, 2, 3, 4, 5 , Zhenhua Sun 1, 2, 3, 4, 5 , Shaopeng Li 1, 2, 3, 4, 5 , Xinjuan Hou 1, 2, 3, 4, 5 , Huiquan Li 1, 2, 3, 4, 5 , Qisheng Wu 6, 7, 8 , Xinguo Xi 6, 7, 8
Dalton Transactions ( IF 3.5 ) Pub Date : 2018-01-29 00:00:00 , DOI: 10.1039/c8dt00033f Aolei Gao 1, 2, 3, 4, 5 , Zhenhua Sun 1, 2, 3, 4, 5 , Shaopeng Li 1, 2, 3, 4, 5 , Xinjuan Hou 1, 2, 3, 4, 5 , Huiquan Li 1, 2, 3, 4, 5 , Qisheng Wu 6, 7, 8 , Xinguo Xi 6, 7, 8
Affiliation
|
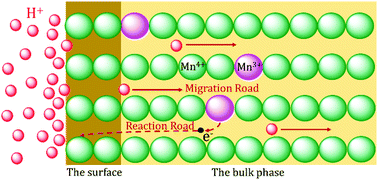
Li1.6Mn1.6O4 is a representative ion sieve material that is used to recover lithium from salt brines and bitterns owing to its high lithium ion adsorption capacity reaching 11.9–44 mg g−1. However, manganese dissolution during acid treatment hinders the industrial application of the material. For investigating the mechanism of manganese dissolution, the precursor Li1.6Mn1.6O4 and ion sieve H1.6Mn1.6O4 were prepared and characterized using X-ray diffraction (XRD), scanning electron microscopy (SEM), Fourier-transform infrared spectroscopy (FT-IR), chemical content analyses, diffuse reflectance infrared Fourier-transform spectroscopy (DRIFTS), and X-ray photoelectron spectroscopy (XPS). The results of XRD, SEM, and FT-IR showed that the bulk phase of Li1.6Mn1.6O4 retained the spinel structure, whereas the lattice diminished during acid treatment. The results of chemical content analyses showed that the bulk phase of Li1.6Mn1.6O4 contained a few trivalent manganese atoms and that the mean valence of manganese in the material increased during acid treatment. DRIFTS and XPS exhibited that the surface of Li1.6Mn1.6O4 was mostly full of tetravalent manganese and retained the spinel structure during acid treatment. In the proposed mechanism of manganese dissolution, an electron of trivalent manganese in the bulk phase transfers to the surface and is captured by tetravalent manganese within the acidic environment. Then, tetravalent manganese is converted to bivalent manganese after acquiring sufficient electrons, and dissolution occurs simultaneously.
中文翻译:

HCl 对Li 1.6 Mn 1.6 O 4离子筛上锰的溶解机理†
Li 1.6 Mn 1.6 O 4是一种代表性的离子筛材料,由于其高的锂离子吸附能力达到11.9–44 mg g -1,因此可用于从盐水和盐卤中回收锂。然而,在酸处理过程中锰的溶解阻碍了该材料的工业应用。为了研究锰的溶解机理,前驱体Li 1.6 Mn 1.6 O 4和离子筛H 1.6 Mn 1.6 O 4使用X射线衍射(XRD),扫描电子显微镜(SEM),傅里叶变换红外光谱(FT-IR),化学成分分析,漫反射红外傅里叶变换光谱(DRIFTS)和X射线制备并表征光电子能谱(XPS)。XRD,SEM和FT-IR结果表明,Li 1.6 Mn 1.6 O 4的体相保留了尖晶石结构,而酸处理过程中的晶格减小。化学含量分析结果表明,Li 1.6 Mn 1.6 O 4的体相包含一些三价锰原子,并且在酸处理过程中材料中锰的平均价增加了。DRIFTS和XPS显示Li 1.6 Mn 1.6 O 4的表面大部分充满四价锰,并且在酸处理期间保留了尖晶石结构。在提出的锰溶解机理中,本体相中的三价锰电子转移到表面并在酸性环境中被四价锰捕获。然后,在获得足够的电子之后,四价锰被转化为二价锰,并且溶解同时发生。
更新日期:2018-01-29
中文翻译:

HCl 对Li 1.6 Mn 1.6 O 4离子筛上锰的溶解机理†
Li 1.6 Mn 1.6 O 4是一种代表性的离子筛材料,由于其高的锂离子吸附能力达到11.9–44 mg g -1,因此可用于从盐水和盐卤中回收锂。然而,在酸处理过程中锰的溶解阻碍了该材料的工业应用。为了研究锰的溶解机理,前驱体Li 1.6 Mn 1.6 O 4和离子筛H 1.6 Mn 1.6 O 4使用X射线衍射(XRD),扫描电子显微镜(SEM),傅里叶变换红外光谱(FT-IR),化学成分分析,漫反射红外傅里叶变换光谱(DRIFTS)和X射线制备并表征光电子能谱(XPS)。XRD,SEM和FT-IR结果表明,Li 1.6 Mn 1.6 O 4的体相保留了尖晶石结构,而酸处理过程中的晶格减小。化学含量分析结果表明,Li 1.6 Mn 1.6 O 4的体相包含一些三价锰原子,并且在酸处理过程中材料中锰的平均价增加了。DRIFTS和XPS显示Li 1.6 Mn 1.6 O 4的表面大部分充满四价锰,并且在酸处理期间保留了尖晶石结构。在提出的锰溶解机理中,本体相中的三价锰电子转移到表面并在酸性环境中被四价锰捕获。然后,在获得足够的电子之后,四价锰被转化为二价锰,并且溶解同时发生。































 京公网安备 11010802027423号
京公网安备 11010802027423号