Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ionic association analysis of LiTDI, LiFSI and LiPF6 in EC/DMC for better Li-ion battery performances
RSC Advances ( IF 3.9 ) Pub Date : 2019-02-06 00:00:00 , DOI: 10.1039/c8ra08430k Christopher L Berhaut 1, 2 , Daniel Lemordant 1 , Patrice Porion 3 , Laure Timperman 1 , Grégory Schmidt 2 , Mériem Anouti 1
RSC Advances ( IF 3.9 ) Pub Date : 2019-02-06 00:00:00 , DOI: 10.1039/c8ra08430k Christopher L Berhaut 1, 2 , Daniel Lemordant 1 , Patrice Porion 3 , Laure Timperman 1 , Grégory Schmidt 2 , Mériem Anouti 1
Affiliation
|
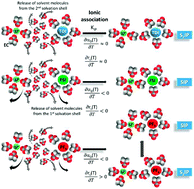
New lithium salts such as lithium bis(fluorosulfonyl)imide (LiFSI) and lithium 4,5-dicyano-2-(trifluoromethyl)imidazole-1-ide (LiTDI) are now challenging lithium hexafluorophosphate (LiPF6), the most used electrolyte salt in commercial Li-ion batteries. Thus it is now important to establish a comparison of these electrolyte components in a standard solvent mixture of ethylene carbonate and dimethyl carbonate (EC/DMC: 50/50 wt%). With this aim, transport properties, such as the ionic conductivity, viscosity and 7Li self-diffusion coefficient have been deeply investigated. Moreover, as these properties are directly linked to the nature of the interionic interactions and ion solvation, a better understanding of the structural properties of electrolytes can be obtained. The Li salt concentration has been varied over the range of 0.1 mol L−1 to 2 mol L−1 at 25 °C and the working temperature from 20 °C to 80 °C at the fixed concentration of 1 mol L−1. Experimental results were used to investigate the temperature dependence of the salt ion-pair (IP) dissociation coefficient (αD) with the help of the Walden rule and the Nernst–Einstein equation. The lithium cation effective solute radius (rLi) has been determined using the Jones–Dole–Kaminsky equation coupled to the Einstein relation for the viscosity of hard spheres in solution and the Stokes–Einstein equation. From the variations of αD and rLi with the temperature, it is inferred that in EC/DMC LiFSI forms solvent-shared ion-pairs (SIP) and that, LiTDI and LiPF6 are likely to form solvent separated ion-pairs (S2IP) or a mixture of SIP and S2IP. From the temperature dependence of αD, thermodynamic parameters such as the standard Gibbs free energy, enthalpy and entropy for the ion-pair formation are obtained. Besides being in agreement with the information provided by the variations of αD and rLi, it is concluded that the ion-pair formation process is exergonic and endothermic for the three salts in EC/DMC.
中文翻译:

在 EC/DMC 中对 LiTDI、LiFSI 和 LiPF6 进行离子关联分析,以获得更好的锂离子电池性能
新的锂盐,例如双(氟磺酰基)亚胺锂 (LiFSI) 和 4,5-二氰基-2-(三氟甲基)咪唑-1-ide (LiTDI) 锂盐现在正在挑战最常用的电解质盐六氟磷酸锂 (LiPF 6 )在商用锂离子电池中。因此,现在重要的是在碳酸亚乙酯和碳酸二甲酯的标准溶剂混合物(EC/DMC:50/50 wt%)中比较这些电解质组分。有了这个目标,传输特性,如离子电导率、粘度和7对锂自扩散系数进行了深入研究。此外,由于这些性质与离子间相互作用和离子溶剂化的性质直接相关,因此可以更好地了解电解质的结构性质。锂盐浓度在 25°C 时在 0.1 mol L -1至 2 mol L -1的范围内变化,在 1 mol L -1的固定浓度下工作温度从 20°C 至 80°C 变化。借助 Walden 规则和 Nernst-Einstein 方程,实验结果用于研究盐离子对 (IP) 解离系数 ( α D ) 的温度依赖性。锂阳离子有效溶质半径(r Li) 已使用 Jones-Dole-Kaminsky 方程与 Einstein 关系对溶液中硬球的粘度和 Stokes-Einstein 方程进行了确定。从α D和 r Li随温度的变化可以推断,在 EC/DMC 中,LiFSI 形成溶剂共享离子对(SIP),LiTDI 和 LiPF 6很可能形成溶剂分离离子对(S 2 IP) 或 SIP 和 S 2 IP 的混合。根据α D的温度依赖性,得到离子对形成的标准吉布斯自由能、焓和熵等热力学参数。除了与变化提供的信息一致α D和 r Li,可以得出结论,对于 EC/DMC 中的三种盐,离子对形成过程是放能和吸热的。
更新日期:2019-02-06
中文翻译:

在 EC/DMC 中对 LiTDI、LiFSI 和 LiPF6 进行离子关联分析,以获得更好的锂离子电池性能
新的锂盐,例如双(氟磺酰基)亚胺锂 (LiFSI) 和 4,5-二氰基-2-(三氟甲基)咪唑-1-ide (LiTDI) 锂盐现在正在挑战最常用的电解质盐六氟磷酸锂 (LiPF 6 )在商用锂离子电池中。因此,现在重要的是在碳酸亚乙酯和碳酸二甲酯的标准溶剂混合物(EC/DMC:50/50 wt%)中比较这些电解质组分。有了这个目标,传输特性,如离子电导率、粘度和7对锂自扩散系数进行了深入研究。此外,由于这些性质与离子间相互作用和离子溶剂化的性质直接相关,因此可以更好地了解电解质的结构性质。锂盐浓度在 25°C 时在 0.1 mol L -1至 2 mol L -1的范围内变化,在 1 mol L -1的固定浓度下工作温度从 20°C 至 80°C 变化。借助 Walden 规则和 Nernst-Einstein 方程,实验结果用于研究盐离子对 (IP) 解离系数 ( α D ) 的温度依赖性。锂阳离子有效溶质半径(r Li) 已使用 Jones-Dole-Kaminsky 方程与 Einstein 关系对溶液中硬球的粘度和 Stokes-Einstein 方程进行了确定。从α D和 r Li随温度的变化可以推断,在 EC/DMC 中,LiFSI 形成溶剂共享离子对(SIP),LiTDI 和 LiPF 6很可能形成溶剂分离离子对(S 2 IP) 或 SIP 和 S 2 IP 的混合。根据α D的温度依赖性,得到离子对形成的标准吉布斯自由能、焓和熵等热力学参数。除了与变化提供的信息一致α D和 r Li,可以得出结论,对于 EC/DMC 中的三种盐,离子对形成过程是放能和吸热的。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号