当前位置:
X-MOL 学术
›
Blood Cancer J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phase I study of the anti-FcRH5 antibody-drug conjugate DFRF4539A in relapsed or refractory multiple myeloma.
Blood Cancer Journal ( IF 12.9 ) Pub Date : 2019-02-04 , DOI: 10.1038/s41408-019-0178-8
A Keith Stewart 1 , Amrita Y Krishnan 2 , Seema Singhal 3 , Ralph V Boccia 4 , Manish R Patel 5, 6 , Ruben Niesvizky 7 , Asher A Chanan-Khan 8 , Sikander Ailawadhi 8 , Jochen Brumm 9 , Kirsten E Mundt 9 , Kyu Hong 9 , Jacqueline McBride 9 , Quyen Shon-Nguyen 9 , Yuanyuan Xiao 9 , Vanitha Ramakrishnan 9 , Andrew G Polson 9 , Divya Samineni 9 , Douglas Leipold 9 , Eric W Humke 9 , James Scott McClellan 9 , Jesus G Berdeja 6
Blood Cancer Journal ( IF 12.9 ) Pub Date : 2019-02-04 , DOI: 10.1038/s41408-019-0178-8
A Keith Stewart 1 , Amrita Y Krishnan 2 , Seema Singhal 3 , Ralph V Boccia 4 , Manish R Patel 5, 6 , Ruben Niesvizky 7 , Asher A Chanan-Khan 8 , Sikander Ailawadhi 8 , Jochen Brumm 9 , Kirsten E Mundt 9 , Kyu Hong 9 , Jacqueline McBride 9 , Quyen Shon-Nguyen 9 , Yuanyuan Xiao 9 , Vanitha Ramakrishnan 9 , Andrew G Polson 9 , Divya Samineni 9 , Douglas Leipold 9 , Eric W Humke 9 , James Scott McClellan 9 , Jesus G Berdeja 6
Affiliation
|
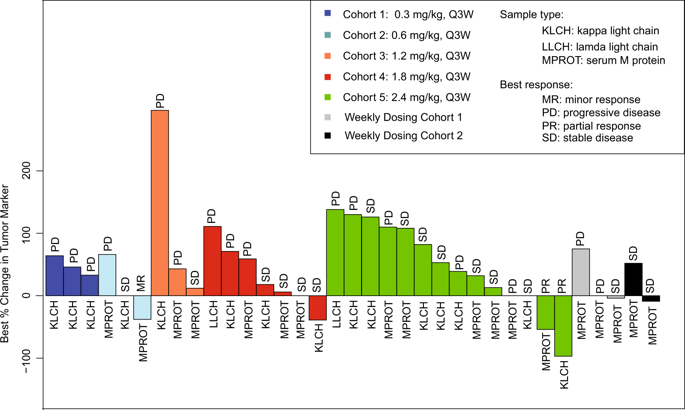
FcRH5 is a cell surface marker enriched on malignant plasma cells when compared to other hematologic malignancies and normal tissues. DFRF4539A is an anti-FcRH5 antibody-drug conjugated to monomethyl auristatin E (MMAE), a potent anti-mitotic agent. This phase I study assessed safety, tolerability, maximum tolerated dose (MTD), anti-tumor activity, and pharmacokinetics of DFRF4539A in patients with relapsed/refractory multiple myeloma. DFRF4539A was administered at 0.3-2.4 mg/kg every 3 weeks or 0.8-1.1 mg/kg weekly as a single-agent by intravenous infusion to 39 patients. Exposure of total antibody and antibody-conjugate-MMAE analytes was linear across the doses tested. There were 37 (95%) adverse events (AEs), 8 (21%) serious AEs, and 15 (39%) AEs ≥ grade 3. Anemia (n = 10, 26%) was the most common AE considered related to DFRF4539A. Two cases of grade 3 acute renal failure were attributed to DFRF4539A. There were no deaths; the MTD was not reached. DFRF4539A demonstrated limited activity in patients at the doses tested with 2 (5%) partial response, 1 (3%) minimal response, 18 (46%) stable disease, and 16 (41%) progressive disease. FcRH5 was confirmed to be expressed and occupied by antibody post-treatment and thus remains a valid myeloma target. Nevertheless, this MMAE-based antibody-drug-conjugate targeting FcRH5 was unsuccessful for myeloma.
中文翻译:

抗FcRH5抗体-药物偶联物DFRF4539A在复发或难治性多发性骨髓瘤中的I期研究。
与其他血液系统恶性肿瘤和正常组织相比,FcRH5是富集在恶性浆细胞上的细胞表面标志物。DFRF4539A是偶联到有效的抗有丝分裂剂单甲基澳瑞他汀E(MMAE)上的抗FcRH5抗体-药物。这项I期研究评估了DFRF4539A在复发/难治性多发性骨髓瘤患者中的安全性,耐受性,最大耐受剂量(MTD),抗肿瘤活性和药代动力学。DFRF4539A每3周以0.3-2.4 mg / kg的单剂量或每周0.8-1.1 mg / kg的单剂通过静脉内输注的方式给予39例患者。在整个测试剂量中,总抗体和抗体-缀合物-MMAE分析物的暴露呈线性关系。≥3级的不良事件(AE)为37(95%),严重不良事件为8(21%),≥3级的不良事件为15(39%)。贫血(n = 10,26%)是被认为与DFRF4539A相关的最常见不良事件。2例3级急性肾衰竭归因于DFRF4539A。没有死亡;未达到MTD。DFRF4539A在以2(5%)部分缓解,1(3%)最小缓解,18(46%)稳定疾病和16(41%)进行性疾病测试的剂量下证明了患者活动受限。FcRH5被证实在抗体后处理中被表达和占据,因此仍然是有效的骨髓瘤靶标。但是,这种靶向FcRH5的基于MMAE的抗体-药物结合物并未成功治疗骨髓瘤。FcRH5被证实在抗体后处理中被表达和占据,因此仍然是有效的骨髓瘤靶标。但是,这种靶向FcRH5的基于MMAE的抗体-药物结合物并未成功治疗骨髓瘤。FcRH5被证实在抗体后处理中被表达和占据,因此仍然是有效的骨髓瘤靶标。但是,这种靶向FcRH5的基于MMAE的抗体-药物结合物并未成功治疗骨髓瘤。
更新日期:2019-07-05
中文翻译:

抗FcRH5抗体-药物偶联物DFRF4539A在复发或难治性多发性骨髓瘤中的I期研究。
与其他血液系统恶性肿瘤和正常组织相比,FcRH5是富集在恶性浆细胞上的细胞表面标志物。DFRF4539A是偶联到有效的抗有丝分裂剂单甲基澳瑞他汀E(MMAE)上的抗FcRH5抗体-药物。这项I期研究评估了DFRF4539A在复发/难治性多发性骨髓瘤患者中的安全性,耐受性,最大耐受剂量(MTD),抗肿瘤活性和药代动力学。DFRF4539A每3周以0.3-2.4 mg / kg的单剂量或每周0.8-1.1 mg / kg的单剂通过静脉内输注的方式给予39例患者。在整个测试剂量中,总抗体和抗体-缀合物-MMAE分析物的暴露呈线性关系。≥3级的不良事件(AE)为37(95%),严重不良事件为8(21%),≥3级的不良事件为15(39%)。贫血(n = 10,26%)是被认为与DFRF4539A相关的最常见不良事件。2例3级急性肾衰竭归因于DFRF4539A。没有死亡;未达到MTD。DFRF4539A在以2(5%)部分缓解,1(3%)最小缓解,18(46%)稳定疾病和16(41%)进行性疾病测试的剂量下证明了患者活动受限。FcRH5被证实在抗体后处理中被表达和占据,因此仍然是有效的骨髓瘤靶标。但是,这种靶向FcRH5的基于MMAE的抗体-药物结合物并未成功治疗骨髓瘤。FcRH5被证实在抗体后处理中被表达和占据,因此仍然是有效的骨髓瘤靶标。但是,这种靶向FcRH5的基于MMAE的抗体-药物结合物并未成功治疗骨髓瘤。FcRH5被证实在抗体后处理中被表达和占据,因此仍然是有效的骨髓瘤靶标。但是,这种靶向FcRH5的基于MMAE的抗体-药物结合物并未成功治疗骨髓瘤。

































 京公网安备 11010802027423号
京公网安备 11010802027423号