当前位置:
X-MOL 学术
›
Adv. Healthcare Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Orally Delivered Antisense Oligodeoxyribonucleotides of TNF‐α via Polysaccharide‐Based Nanocomposites Targeting Intestinal Inflammation
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2019-02-04 , DOI: 10.1002/adhm.201801389 Bingchao Duan 1 , Mengxia Li 1 , Ying Sun 1 , Siwei Zou 1 , Xiaojuan Xu 1
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2019-02-04 , DOI: 10.1002/adhm.201801389 Bingchao Duan 1 , Mengxia Li 1 , Ying Sun 1 , Siwei Zou 1 , Xiaojuan Xu 1
Affiliation
|
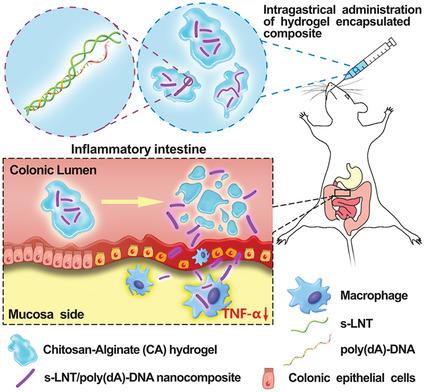
Tumor necrosis factor alpha (TNF‐α) is usually regarded as a potential target for inflammatory bowel disease therapy. Herein, a promising strategy for effective delivery of phosphorothioated antisense oligodeoxyribonucleotide of TNF‐α (PS‐ATNF‐α), targeting the intestinal inflammation based on the interaction of the single chain of triple helical β‐glucan (s‐LNT) with poly‐deoxyadenylic acid [poly(dA)], and the colon‐specific degradation of chitosan‐alginate (CA) hydrogel, is reported. The target gene of PS‐ATNF‐α, with a poly(dA) tail through a disulfide bond (–SS–), interacts with s‐LNT to form a rod‐like nanocomposite of s‐LNT/poly(dA)–SS–PS‐ATNF‐α, which significantly inhibits lipopolysaccharide (LPS)‐induced TNF‐α at the protein level by 38.2% and mRNA level by 48.9% in RAW264.7 macrophages. The nanocomposites carried by the CA hydrogel with the loading amount of 83.5% are then orally administered and specifically released to the inflamed intestine, followed by internalization into intestinal cells such as macrophages, to reduce TNF‐α production by 36.4% and dextran sulfate sodium‐induced inflammation by decreasing myeloperoxidase and malondialdehyde. This study defines a new strategy for the oral delivery of antisense oligonucleotides to attenuate inflammatory response, demonstrating a notable potential for clinical applications in intestine‐inflammation‐targeted therapy.
中文翻译:

通过靶向肠道炎症的基于多糖的纳米复合材料口服递送TNF-α的反义寡核苷酸。
肿瘤坏死因子α(TNF-α)通常被认为是炎症性肠病治疗的潜在靶标。本文提出了一种有效递送TNF-α的硫代磷酸反义寡聚脱氧核糖核苷酸(PS-ATNF-α)的有前途的策略,其基于三螺旋β-葡聚糖(s-LNT)的单链与多聚β-葡聚糖的相互作用来靶向肠道炎症。据报道,脱氧腺苷酸[poly(dA)]和壳聚糖-藻酸盐(CA)水凝胶的结肠特异性降解。PS-ATNF-α的靶基因具有通过二硫键(–SS–)的聚(dA)尾巴,与s–LNT相互作用形成s–LNT / poly(dA)–SS的杆状纳米复合材料-PS-ATNF-α,在RAW264.7巨噬细胞中,在蛋白质水平上显着抑制脂多糖(LPS)诱导的TNF-α含量达38.2%,mRNA水平达48.9%。然后口服CA水凝胶载带的纳米复合材料,负载量为83.5%,并专门释放到发炎的肠中,然后内化到肠细胞(如巨噬细胞)中,以减少TNF-α产生36.4%和右旋糖酐硫酸钠。通过降低髓过氧化物酶和丙二醛诱导炎症。这项研究确定了口服反义寡核苷酸减弱炎症反应的新策略,证明了在针对肠道炎症的治疗中的临床应用具有显着潜力。4%和硫酸右旋糖酐钠通过减少髓过氧化物酶和丙二醛引起的炎症。这项研究确定了口服反义寡核苷酸减弱炎症反应的新策略,证明了在针对肠道炎症的治疗中的临床应用具有显着潜力。4%和硫酸右旋糖酐钠通过减少髓过氧化物酶和丙二醛引起的炎症。这项研究确定了口服反义寡核苷酸减弱炎症反应的新策略,证明了在针对肠道炎症的治疗中临床应用的显着潜力。
更新日期:2019-02-04
中文翻译:

通过靶向肠道炎症的基于多糖的纳米复合材料口服递送TNF-α的反义寡核苷酸。
肿瘤坏死因子α(TNF-α)通常被认为是炎症性肠病治疗的潜在靶标。本文提出了一种有效递送TNF-α的硫代磷酸反义寡聚脱氧核糖核苷酸(PS-ATNF-α)的有前途的策略,其基于三螺旋β-葡聚糖(s-LNT)的单链与多聚β-葡聚糖的相互作用来靶向肠道炎症。据报道,脱氧腺苷酸[poly(dA)]和壳聚糖-藻酸盐(CA)水凝胶的结肠特异性降解。PS-ATNF-α的靶基因具有通过二硫键(–SS–)的聚(dA)尾巴,与s–LNT相互作用形成s–LNT / poly(dA)–SS的杆状纳米复合材料-PS-ATNF-α,在RAW264.7巨噬细胞中,在蛋白质水平上显着抑制脂多糖(LPS)诱导的TNF-α含量达38.2%,mRNA水平达48.9%。然后口服CA水凝胶载带的纳米复合材料,负载量为83.5%,并专门释放到发炎的肠中,然后内化到肠细胞(如巨噬细胞)中,以减少TNF-α产生36.4%和右旋糖酐硫酸钠。通过降低髓过氧化物酶和丙二醛诱导炎症。这项研究确定了口服反义寡核苷酸减弱炎症反应的新策略,证明了在针对肠道炎症的治疗中的临床应用具有显着潜力。4%和硫酸右旋糖酐钠通过减少髓过氧化物酶和丙二醛引起的炎症。这项研究确定了口服反义寡核苷酸减弱炎症反应的新策略,证明了在针对肠道炎症的治疗中的临床应用具有显着潜力。4%和硫酸右旋糖酐钠通过减少髓过氧化物酶和丙二醛引起的炎症。这项研究确定了口服反义寡核苷酸减弱炎症反应的新策略,证明了在针对肠道炎症的治疗中临床应用的显着潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号