European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-02-02 , DOI: 10.1016/j.ejmech.2019.01.076 Laurita Boff , Jennifer Munkert , Flaviano Melo Ottoni , Naira Fernanda Zanchett Schneider , Gabriela Silva Ramos , Wolfgang Kreis , Saulo Fernandes de Andrade , José Dias de Souza Filho , Fernão Castro Braga , Ricardo José Alves , Rodrigo Maia de Pádua , Cláudia Maria Oliveira Simões
|
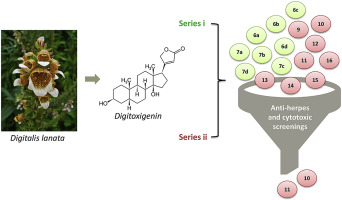
In recent years, new therapeutic possibilities were proposed for cardiac glycosides traditionally used to treat heart diseases, such as anticancer and antiviral activities. In this sense, this work aimed to synthesize the readily accessible 3β-azido-3-deoxydigitoxigenin (5) from digitoxigenin (1). Two new series of compounds were obtained from derivative (5): (i) O-glycosyl trizols through click chemistry with propargyl glycosides; and (ii) compounds substituted in the alpha carbonyl position with different residues linked via an amino-group. All obtained derivatives have their chemical structures confirmed, and their anti-herpes (against HSV-types 1 and 2 replication) and cytotoxic (against PC3, A549, HCT-8 and LNCaP cell lines) activities evaluated. Compounds 10 and 11 exhibited the most promising results against HSV-1 (KOS and 29-R strains) and HSV-2 (333 strain) replication with SI values > 1000. Both compounds were also the most cytotoxic for the human cancer cell lines tested with IC50 values similar to those of paclitaxel. They also presented reduced toxicity toward non-cancerous cell lines (MRC-5 and HGF cells). Promising compounds were tested in regard to their ability to inhibit Na+/K+-ATPase. The inhibition rate correlates suitably with the bioactivity demonstrated by those both compounds against the different human cancer cells tested as well as against HSV replication. Moreover, the results showed that specific chemical features of compound 10 and 11 influenced the bioactivities tested. In summary, it was possible to obtain novel digitoxigenin-derivatives with remarkable cytotoxic and anti-herpes activities as well as low toxicity and high selectivity. In this way, they could be considered potential molecules for the development of new drugs.
中文翻译:

新型半合成洋地黄毒苷衍生物的潜在抗疱疹和细胞毒性作用
近年来,对于传统上用于治疗诸如抗癌和抗病毒活性的心脏病的强心苷提出了新的治疗可能性。从这个意义上讲,这项工作旨在从洋地黄毒苷原(1)合成容易获得的3β-叠氮基-3-脱氧指氧黄体生成黄素(5 )。从衍生物(5)获得了两个新系列的化合物:(i)O通过与炔丙基糖苷的点击化学反应制得-糖基三唑;(ii)在α羰基位上被通过氨基连接的不同残基取代的化合物。所有获得的衍生物均已确认其化学结构,并评估了其抗疱疹(针对HSV 1型和2型复制)和细胞毒性(针对PC3,A549,HCT-8和LNCaP细胞系)的活性。化合物10和11表现出对HSV-1(KOS和29-R株)和HSV-2(333株)复制最有希望的结果,SI值>1000。这两种化合物对于所测试的人类癌细胞系也是最具细胞毒性的带有IC 50值与紫杉醇相似。他们还表现出对非癌细胞系(MRC-5和HGF细胞)毒性降低。测试了有前途的化合物抑制Na + / K + -ATPase的能力。抑制率与这两种化合物显示出的针对所测试的不同人类癌细胞以及针对HSV复制的生物活性相关。此外,结果表明化合物10和11的特定化学特征影响了测试的生物活性。总之,可以获得具有显着的细胞毒性和抗疱疹活性以及低毒性和高选择性的新型洋地黄毒苷衍生物。这样,它们可以被认为是开发新药的潜在分子。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号