当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis and biological evaluation of inhibitors of cathepsin K on dedifferentiated chondrocytes.
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2019-02-02 , DOI: 10.1016/j.bmc.2019.02.003 Xiao-Yu Yuan 1 , Zhongyuan Ren 2 , Yuqing Wu 2 , Carole Bougault 3 , Leyre Brizuela 3 , David Magne 3 , René Buchet 3 , Saida Mebarek 3
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2019-02-02 , DOI: 10.1016/j.bmc.2019.02.003 Xiao-Yu Yuan 1 , Zhongyuan Ren 2 , Yuqing Wu 2 , Carole Bougault 3 , Leyre Brizuela 3 , David Magne 3 , René Buchet 3 , Saida Mebarek 3
Affiliation
|
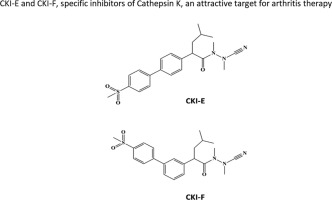
Selective proteinase inhibitors have demonstrated utility in the investigation of cartilage degeneration mechanisms and may have clinical use in the management of osteoarthritis. The cysteine protease cathepsin K (CatK) is an attractive target for arthritis therapy. Here we report the synthesis of two cathepsin K inhibitors (CKIs): racemic azanitrile derivatives CKI-E and CKI-F, which have better inhibition properties on CatK than the commercial inhibitor odanacatib (ODN). Their IC50 values and inhibition constants (Ki) have been determined in vitro. Inhibitors demonstrate differential selectivity for CatK over cathepsin B, L and S in vitro, with Ki amounting to 1.14 and 7.21 nM respectively. We analyzed the effect of these racemic inhibitors on viability in different cell types. The human osteoblast-like cell line MG63, MOVAS cells (a murine vascular smooth muscle cell line) or murine primary chondrocytes, were treated either with CKI-E or with CKI-F, which were not toxic at doses of up to 5 µM. Primary chondrocytes subjected to several passages were used as a model of phenotypic loss of articular chondrocytes, occurring in osteoarthritic cartilage. The efficiency of CKIs regarding CatK inhibition and their specificity over other proteases were validated in primary chondrocytes subjected to several passages. Racemic CKI-E and CKI-F at 0.1 and 1 µM significantly inhibited CatK activity in dedifferentiated chondrocytes, even better than the commercial CatK inhibitor ODN. The enzymatic activity of other proteases such as matrix metalloproteinases or aggrecanases were not affected. Taken together, these findings support the possibility to design CatK inhibitors for preventing cartilage degradation in different pathologies.
中文翻译:

组织蛋白酶K抑制剂对去分化软骨细胞的设计,合成和生物学评估。
选择性蛋白酶抑制剂已被证明可用于软骨退变机制的研究,并可能在骨关节炎的治疗中具有临床用途。半胱氨酸蛋白酶组织蛋白酶K(CatK)是关节炎治疗的一个有吸引力的目标。在这里,我们报告了两种组织蛋白酶K抑制剂(CKIs)的合成:外消旋氮杂腈衍生物CKI-E和CKI-F,与商业抑制剂odanacatib(ODN)相比,它们对CatK具有更好的抑制性能。它们的IC50值和抑制常数(Ki)已在体外确定。抑制剂在体外表现出对CatK的选择性高于组织蛋白酶B,L和S的选择性,Ki分别为1.14和7.21 nM。我们分析了这些外消旋抑制剂对不同细胞类型生存力的影响。人成骨样细胞系MG63,用CKI-E或CKI-F处理MOVAS细胞(鼠血管平滑肌细胞系)或鼠原代软骨细胞,剂量不超过5 µM时无毒。经历几次传代的原代软骨细胞被用作骨关节炎软骨中发生的关节软骨细胞表型丧失的模型。CKIs对CatK抑制的效率及其对其他蛋白酶的特异性已在经历数次传代的原代软骨细胞中得到验证。0.1和1 µM的外消旋CKI-E和CKI-F显着抑制去分化软骨细胞中的CatK活性,甚至比市售的CatK抑制剂ODN更好。其他蛋白酶(例如基质金属蛋白酶或软骨聚集蛋白聚糖酶)的酶促活性不受影响。在一起
更新日期:2019-02-02
中文翻译:

组织蛋白酶K抑制剂对去分化软骨细胞的设计,合成和生物学评估。
选择性蛋白酶抑制剂已被证明可用于软骨退变机制的研究,并可能在骨关节炎的治疗中具有临床用途。半胱氨酸蛋白酶组织蛋白酶K(CatK)是关节炎治疗的一个有吸引力的目标。在这里,我们报告了两种组织蛋白酶K抑制剂(CKIs)的合成:外消旋氮杂腈衍生物CKI-E和CKI-F,与商业抑制剂odanacatib(ODN)相比,它们对CatK具有更好的抑制性能。它们的IC50值和抑制常数(Ki)已在体外确定。抑制剂在体外表现出对CatK的选择性高于组织蛋白酶B,L和S的选择性,Ki分别为1.14和7.21 nM。我们分析了这些外消旋抑制剂对不同细胞类型生存力的影响。人成骨样细胞系MG63,用CKI-E或CKI-F处理MOVAS细胞(鼠血管平滑肌细胞系)或鼠原代软骨细胞,剂量不超过5 µM时无毒。经历几次传代的原代软骨细胞被用作骨关节炎软骨中发生的关节软骨细胞表型丧失的模型。CKIs对CatK抑制的效率及其对其他蛋白酶的特异性已在经历数次传代的原代软骨细胞中得到验证。0.1和1 µM的外消旋CKI-E和CKI-F显着抑制去分化软骨细胞中的CatK活性,甚至比市售的CatK抑制剂ODN更好。其他蛋白酶(例如基质金属蛋白酶或软骨聚集蛋白聚糖酶)的酶促活性不受影响。在一起






























 京公网安备 11010802027423号
京公网安备 11010802027423号