当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Optimization of 5,6,7,8-tetrahydropyrido[4,3-d]pyrimidines to generate a highly selective PI3Kδ inhibitor.
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2019-02-01 , DOI: 10.1016/j.bmc.2019.02.001
Toshihiro Hamajima 1 , Fumie Takahashi 1 , Koji Kato 1 , Yukihito Sugano 1 , Susumu Yamaki 1 , Daisuke Suzuki 1 , Ayako Moritomo 1 , Satoshi Kubo 1 , Koji Nakamura 1 , Kaoru Yamagami 1 , Koji Yokoo 1 , Hidehiko Fukahori 1
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2019-02-01 , DOI: 10.1016/j.bmc.2019.02.001
Toshihiro Hamajima 1 , Fumie Takahashi 1 , Koji Kato 1 , Yukihito Sugano 1 , Susumu Yamaki 1 , Daisuke Suzuki 1 , Ayako Moritomo 1 , Satoshi Kubo 1 , Koji Nakamura 1 , Kaoru Yamagami 1 , Koji Yokoo 1 , Hidehiko Fukahori 1
Affiliation
|
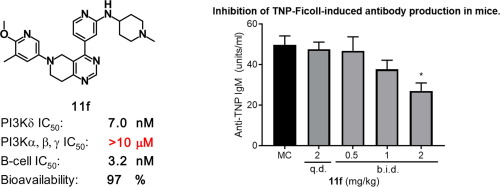
Chemical optimization of the 5,6,7,8-tetrahydropyrido[4,3-d]pyrimidine (THPP) scaffold was conducted with a focus on cellular potency while maintaining high selectivity against PI3K isoforms. Compound 11f was identified as a potent, highly selective and orally available PI3Kδ inhibitor. In addition, 11f exhibited efficacy in an in vivo antibody production model. The desirable drug-like properties and in vivo efficacy of 11f suggest its potential as a drug candidate for the treatment of autoimmune diseases and leukocyte malignancies.
中文翻译:

优化5,6,7,8-四氢吡啶并[4,3-d]嘧啶以产生高度选择性的PI3Kδ抑制剂。
对5,6,7,8-四氢吡啶并[4,3-d]嘧啶(THPP)支架进行化学优化,重点是细胞效能,同时保持对PI3K同工型的高选择性。化合物11f被确定为有效的,高度选择性的和口服可得的PI3Kδ抑制剂。此外,11f在体内抗体产生模型中显示出功效。11f的理想药物样特性和体内功效表明其作为治疗自身免疫性疾病和白细胞恶性肿瘤的候选药物的潜力。
更新日期:2019-02-01
中文翻译:

优化5,6,7,8-四氢吡啶并[4,3-d]嘧啶以产生高度选择性的PI3Kδ抑制剂。
对5,6,7,8-四氢吡啶并[4,3-d]嘧啶(THPP)支架进行化学优化,重点是细胞效能,同时保持对PI3K同工型的高选择性。化合物11f被确定为有效的,高度选择性的和口服可得的PI3Kδ抑制剂。此外,11f在体内抗体产生模型中显示出功效。11f的理想药物样特性和体内功效表明其作为治疗自身免疫性疾病和白细胞恶性肿瘤的候选药物的潜力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号