当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rapid nickel(ii)-promoted cysteine S-arylation with arylboronic acids†
Chemical Communications ( IF 4.3 ) Pub Date : 2019-02-04 00:00:00 , DOI: 10.1039/c9cc00159j Kengo Hanaya 1, 2, 3 , Jun Ohata 1, 2, 3 , Mary K. Miller 1, 2, 3 , Alicia E. Mangubat-Medina 1, 2, 3 , Michael J. Swierczynski 1, 2, 3 , David C. Yang 1, 2, 3 , Reece M. Rosenthal 1, 2, 3 , Brian V. Popp 3, 4, 5 , Zachary T. Ball 1, 2, 3
Chemical Communications ( IF 4.3 ) Pub Date : 2019-02-04 00:00:00 , DOI: 10.1039/c9cc00159j Kengo Hanaya 1, 2, 3 , Jun Ohata 1, 2, 3 , Mary K. Miller 1, 2, 3 , Alicia E. Mangubat-Medina 1, 2, 3 , Michael J. Swierczynski 1, 2, 3 , David C. Yang 1, 2, 3 , Reece M. Rosenthal 1, 2, 3 , Brian V. Popp 3, 4, 5 , Zachary T. Ball 1, 2, 3
Affiliation
|
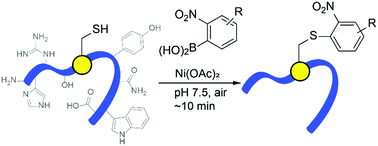
S-Arylation of cysteine residues is an increasingly powerful tool for site-specific modification of proteins, providing novel structure and electronic perturbation. The present work demonstrates an operationally-simple cysteine arylation reaction 2-nitro-substituted arylboronic acids, promoted by a simple nickel(II) salt. The process exhibits strikingly fast reaction rates under physiological conditions in purely aqueous media with excellent selectivity toward cysteine residues. Cysteine arylation of natural proteins and peptides allows attachment of useful reactive handles for stapling, imaging, or further conjugation.
中文翻译:

快速镍(ii)促进的半胱氨酸S与芳基硼酸的芳基化†
半胱氨酸残基的S-酰化是蛋白质位点特异性修饰的一种越来越强大的工具,它提供了新颖的结构和电子扰动。本工作证明了操作上简单的半胱氨酸芳基化反应2-硝基取代的芳基硼酸,由简单的镍(II)盐促进。该方法在生理条件下,在纯水介质中对半胱氨酸残基具有优异的选择性,显示出惊人的快速反应速率。天然蛋白质和肽的半胱氨酸芳基化反应可以连接有用的反应性手柄,用于装订,成像或进一步偶联。
更新日期:2019-02-04
中文翻译:

快速镍(ii)促进的半胱氨酸S与芳基硼酸的芳基化†
半胱氨酸残基的S-酰化是蛋白质位点特异性修饰的一种越来越强大的工具,它提供了新颖的结构和电子扰动。本工作证明了操作上简单的半胱氨酸芳基化反应2-硝基取代的芳基硼酸,由简单的镍(II)盐促进。该方法在生理条件下,在纯水介质中对半胱氨酸残基具有优异的选择性,显示出惊人的快速反应速率。天然蛋白质和肽的半胱氨酸芳基化反应可以连接有用的反应性手柄,用于装订,成像或进一步偶联。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号