当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Non-equilibrium crystallization pathways of manganese oxides in aqueous solution.
Nature Communications ( IF 14.7 ) Pub Date : 2019-02-04 , DOI: 10.1038/s41467-019-08494-6 Wenhao Sun 1 , Daniil A Kitchaev 2 , Denis Kramer 3 , Gerbrand Ceder 1, 2, 4
Nature Communications ( IF 14.7 ) Pub Date : 2019-02-04 , DOI: 10.1038/s41467-019-08494-6 Wenhao Sun 1 , Daniil A Kitchaev 2 , Denis Kramer 3 , Gerbrand Ceder 1, 2, 4
Affiliation
|
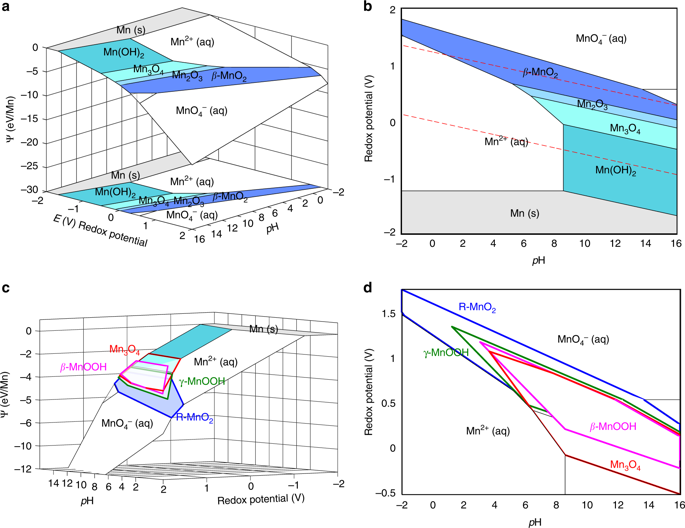
Aqueous precipitation of transition metal oxides often proceeds through non-equilibrium phases, whose appearance cannot be anticipated from traditional phase diagrams. Without a precise understanding of which metastable phases form, or their lifetimes, targeted synthesis of specific metal oxides can become a trial-and-error process. Here, we construct a theoretical framework to reveal the nanoscale and metastable energy landscapes of Pourbaix (E-pH) diagrams, providing quantitative insights into the size-dependent thermodynamics of metastable oxide nucleation and growth in water. By combining this framework with classical nucleation theory, we interrogate how solution conditions influence the multistage oxidation pathways of manganese oxides. We calculate that even within the same stability region of a Pourbaix diagram, subtle variations in pH and redox potential can redirect a non-equilibrium crystallization pathway through different metastable intermediates. Our theoretical framework offers a predictive platform to navigate through the thermodynamic and kinetic energy landscape towards the rational synthesis of target materials.
中文翻译:

锰氧化物在水溶液中的非平衡结晶途径。
过渡金属氧化物的水沉淀通常通过非平衡相进行,从传统相图中无法预期其出现。如果不精确了解形成哪种亚稳相或它们的寿命,特定金属氧化物的目标合成可能会成为一个反复试验的过程。在这里,我们构建了一个理论框架来揭示Pourbaix图(E-pH)图的纳米级和亚稳态能图,为亚稳态氧化物成核和水中生长的尺寸依赖性热力学提供了定量的见解。通过将该框架与经典的成核理论相结合,我们研究了固溶条件如何影响锰氧化物的多级氧化途径。我们计算出,即使在Pourbaix图的相同稳定性区域内,pH和氧化还原电位的细微变化可以通过不同的亚稳中间体重定向非平衡结晶途径。我们的理论框架提供了一个预测平台,可在热力学和动能格局中导航,以实现目标材料的合理合成。
更新日期:2019-02-05
中文翻译:

锰氧化物在水溶液中的非平衡结晶途径。
过渡金属氧化物的水沉淀通常通过非平衡相进行,从传统相图中无法预期其出现。如果不精确了解形成哪种亚稳相或它们的寿命,特定金属氧化物的目标合成可能会成为一个反复试验的过程。在这里,我们构建了一个理论框架来揭示Pourbaix图(E-pH)图的纳米级和亚稳态能图,为亚稳态氧化物成核和水中生长的尺寸依赖性热力学提供了定量的见解。通过将该框架与经典的成核理论相结合,我们研究了固溶条件如何影响锰氧化物的多级氧化途径。我们计算出,即使在Pourbaix图的相同稳定性区域内,pH和氧化还原电位的细微变化可以通过不同的亚稳中间体重定向非平衡结晶途径。我们的理论框架提供了一个预测平台,可在热力学和动能格局中导航,以实现目标材料的合理合成。






























 京公网安备 11010802027423号
京公网安备 11010802027423号