Nature Communications ( IF 14.7 ) Pub Date : 2019-02-04 , DOI: 10.1038/s41467-019-08447-z
Lei Zhang , Shao-Hua Xiang , Jun Wang , Jian Xiao , Jun-Qi Wang , Bin Tan
|
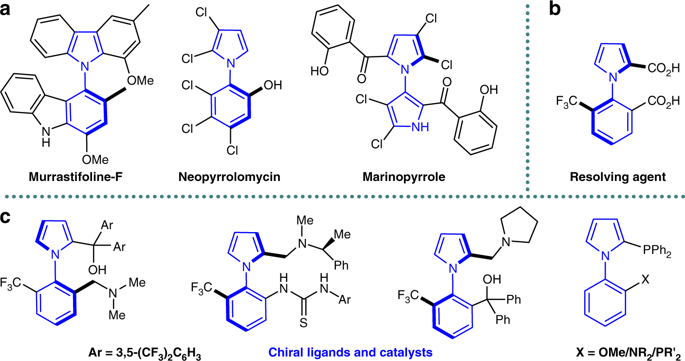
Axially chiral arylpyrroles are key components of pharmaceuticals and natural products as well as chiral catalysts and ligands for asymmetric transformations. However, the catalytic enantioselective construction of optically active arylpyrroles remains a formidable challenge. Here we disclose a highly efficient strategy to access enantioenriched axially chiral arylpyrroles by means of organocatalytic atroposelective desymmetrization and kinetic resolution. Depending on the remote control of chiral catalyst, the arylpyrroles were obtained in high yields and excellent enantioselectivities under mild reaction conditions. This strategy tolerates a wide range of functional groups, providing a facile avenue to approach axially chiral arylpyrroles from simple and readily available starting materials. Selected arylpyrrole products proved to be efficient chiral ligands in asymmetric catalysis and also important precursors for further synthetic transformations into highly functionalized pyrroles with potential bioactivity, especially the axially chiral fully substituted arylpyrroles.
中文翻译:

磷酸催化的轴向手性芳基吡咯的对映选择性结构
轴向手性芳基吡咯是药物和天然产物以及不对称转化的手性催化剂和配体的关键组分。然而,旋光性芳基吡咯的催化对映选择性结构仍然是一个艰巨的挑战。在这里,我们公开了一种通过有机催化的对位选择性脱对称和动力学拆分来获得对映体富集的轴向手性芳基吡咯的高效策略。根据手性催化剂的远程控制,在温和的反应条件下,可以高收率和优异的对映选择性获得芳基吡咯。该策略可耐受各种官能团,为从简单易得的起始原料接近轴向手性芳基吡咯提供了简便的途径。

































 京公网安备 11010802027423号
京公网安备 11010802027423号