Nature Communications ( IF 14.7 ) Pub Date : 2019-02-04 , DOI: 10.1038/s41467-019-08448-y Meaghan A. Valliere , Tyler P. Korman , Nicholas B. Woodall , Gregory A. Khitrov , Robert E. Taylor , David Baker , James U. Bowie
|
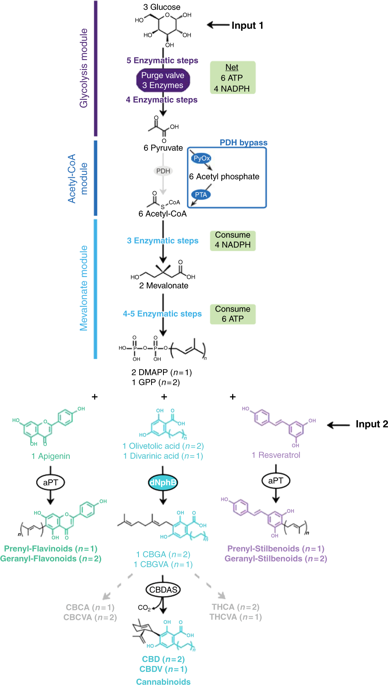
Prenylation of natural compounds adds structural diversity, alters biological activity, and enhances therapeutic potential. Because prenylated compounds often have a low natural abundance, alternative production methods are needed. Metabolic engineering enables natural product biosynthesis from inexpensive biomass, but is limited by the complexity of secondary metabolite pathways, intermediate and product toxicities, and substrate accessibility. Alternatively, enzyme catalyzed prenyl transfer provides excellent regio- and stereo-specificity, but requires expensive isoprenyl pyrophosphate substrates. Here we develop a flexible cell-free enzymatic prenylating system that generates isoprenyl pyrophosphate substrates from glucose to prenylate an array of natural products. The system provides an efficient route to cannabinoid precursors cannabigerolic acid (CBGA) and cannabigerovarinic acid (CBGVA) at >1 g/L, and a single enzymatic step converts the precursors into cannabidiolic acid (CBDA) and cannabidivarinic acid (CBDVA). Cell-free methods may provide a powerful alternative to metabolic engineering for chemicals that are hard to produce in living organisms.
中文翻译:

一个无细胞平台,用于天然产物的异戊酸酯化及其在大麻素生产中的应用
天然化合物的异戊烯基化增加了结构多样性,改变了生物活性,并增强了治疗潜力。由于异戊二烯基化合物的自然丰度通常较低,因此需要其他生产方法。代谢工程可以从廉价的生物质中合成天然产物,但受到次生代谢途径的复杂性,中间产物毒性和底物可及性的限制。或者,酶催化的异戊烯基转移可提供出色的区域和立体特异性,但需要昂贵的异戊二烯基焦磷酸底物。在这里,我们开发了一种灵活的无细胞酶促烯丙基化系统,该系统可从葡萄糖生成异戊烯基焦磷酸底物,从而对一系列天然产物进行烯丙基化。该系统提供了> 1 g / L的大麻素前体大麻双酚酸(CBGA)和大麻双戊二酸(CBGVA)的有效途径,并且一个酶促步骤将前体转化为大麻二酚酸(CBDA)和大麻二炔酸(CBDVA)。对于在活生物体中难以产生的化学物质,无细胞方法可以为代谢工程提供强大的替代方法。






























 京公网安备 11010802027423号
京公网安备 11010802027423号