当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeted Delivery of a γ-Glutamyl Transpeptidase Activatable Near-Infrared-Fluorescent Probe for Selective Cancer Imaging
Analytical Chemistry ( IF 6.7 ) Pub Date : 2018-02-07 00:00:00 , DOI: 10.1021/acs.analchem.7b05022 Zhiliang Luo 1 , Zheng Huang 1 , Ke Li 2 , Yidan Sun 1 , Jianguo Lin 2 , Deju Ye 1 , Hong-Yuan Chen 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2018-02-07 00:00:00 , DOI: 10.1021/acs.analchem.7b05022 Zhiliang Luo 1 , Zheng Huang 1 , Ke Li 2 , Yidan Sun 1 , Jianguo Lin 2 , Deju Ye 1 , Hong-Yuan Chen 1
Affiliation
|
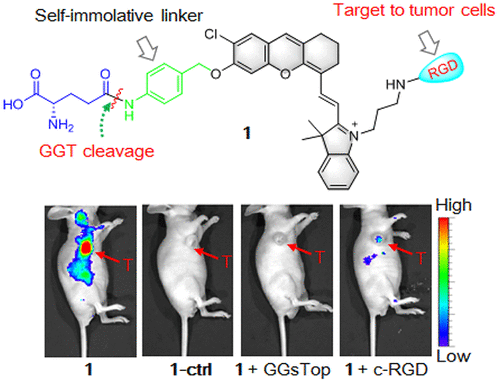
The noninvasive and specific detection of cancer cells in living subjects has been essential for the success of cancer diagnoses and treatments. Herein, we report a strategy of combining an αvβ3-integrin-receptor-targetable ligand, c-RGD, with the γ-glutamyl transpeptidase (GGT)-recognizable substrate, γ-glutamate (γ-Glu), to develop a tumor-targeting and GGT-activatable near-infrared (NIR)-fluorescent probe for the noninvasive imaging of tumors in living mice. We demonstrated that the probe’s fluorescence was off initially, but when the γ-Glu in the probe was specifically cleaved by GGT, the fluorescent product was released and could be selectively taken up by U87MG-tumor cells via αvβ3-receptor-mediated endocytosis. Remarkably, enhanced intracellular NIR fluorescence distributed mainly in the lysosomes was observed in the tumor cells only, showing that the probe was capable of differentiating the tumor cells from the GGT-positive, αvβ3-deficient normal cells. Moreover, the probe also showed a high selectivity for the real-time and noninvasive detection of GGT activity in xenograft U87MG tumors following iv administration. This study reveals the advantage of using a combination of receptor-mediated cell uptake and molecular-target-triggered activation to design molecular probes for improved cancer imaging, which could facilitate effective cancer diagnoses.
中文翻译:

针对选择性癌症成像的γ-谷氨酰转肽酶激活的近红外荧光探针的靶向递送
活体受试者中癌细胞的非侵入性和特异性检测对于癌症诊断和治疗的成功至关重要。此,我们报告的α相结合的策略v β 3整合素受体靶向配体,C-RGD,用γ谷氨酰转肽酶(GGT)-recognizable基板,γ谷氨酸(γ-GLU),开发出靶向肿瘤和可激活GGT的近红外(NIR)荧光探针用于活体小鼠肿瘤的非侵入性成像。我们证明了该探针的荧光被关最初,但是当γ-Glu浓度的探针特异性通过GGT裂解,荧光产物被释放,可经由α被选择性地吸收由U87MG肿瘤细胞v β 3受体介导的内吞作用。值得注意的是,增强的主要分布在细胞内的溶酶体NIR荧光仅在肿瘤细胞中观察到,表明探针是能够从GGT阳性,α分化的肿瘤细胞中v β 3个缺陷型的正常细胞。此外,该探针还显示出对静脉内给药后异种移植U87MG肿瘤中GGT活性的实时和非侵入性检测的高选择性。这项研究揭示了结合使用受体介导的细胞摄取和分子靶点触发的激活来设计分子探针以改善癌症影像的优势,这可以促进有效的癌症诊断。
更新日期:2018-02-07
中文翻译:

针对选择性癌症成像的γ-谷氨酰转肽酶激活的近红外荧光探针的靶向递送
活体受试者中癌细胞的非侵入性和特异性检测对于癌症诊断和治疗的成功至关重要。此,我们报告的α相结合的策略v β 3整合素受体靶向配体,C-RGD,用γ谷氨酰转肽酶(GGT)-recognizable基板,γ谷氨酸(γ-GLU),开发出靶向肿瘤和可激活GGT的近红外(NIR)荧光探针用于活体小鼠肿瘤的非侵入性成像。我们证明了该探针的荧光被关最初,但是当γ-Glu浓度的探针特异性通过GGT裂解,荧光产物被释放,可经由α被选择性地吸收由U87MG肿瘤细胞v β 3受体介导的内吞作用。值得注意的是,增强的主要分布在细胞内的溶酶体NIR荧光仅在肿瘤细胞中观察到,表明探针是能够从GGT阳性,α分化的肿瘤细胞中v β 3个缺陷型的正常细胞。此外,该探针还显示出对静脉内给药后异种移植U87MG肿瘤中GGT活性的实时和非侵入性检测的高选择性。这项研究揭示了结合使用受体介导的细胞摄取和分子靶点触发的激活来设计分子探针以改善癌症影像的优势,这可以促进有效的癌症诊断。































 京公网安备 11010802027423号
京公网安备 11010802027423号