Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Concentration of Organosols of Silver Nanoparticles Stabilized by AOT: Emulsion Versus Microemulsion
Langmuir ( IF 3.7 ) Pub Date : 2018-02-12 00:00:00 , DOI: 10.1021/acs.langmuir.7b04071
Alexander I. Bulavchenko 1 , Aida T. Arymbaeva 1 , Marina G. Demidova 1 , Pavel S. Popovetskiy 1 , Pavel E. Plyusnin 1 , Olga A. Bulavchenko 2
Langmuir ( IF 3.7 ) Pub Date : 2018-02-12 00:00:00 , DOI: 10.1021/acs.langmuir.7b04071
Alexander I. Bulavchenko 1 , Aida T. Arymbaeva 1 , Marina G. Demidova 1 , Pavel S. Popovetskiy 1 , Pavel E. Plyusnin 1 , Olga A. Bulavchenko 2
Affiliation
|
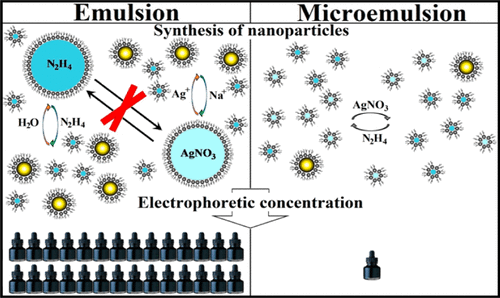
In this work, we tried to combine the advantages of microemulsion and emulsion synthesis to obtain stable concentrated organosols of Ag nanoparticles, promising liquid-phase materials. Starting reagents were successively introduced into a micellar solution of sodium bis-(2-ethylhexyl)sulfosuccinate (AOT) in n-decane in the dynamic reverse emulsion mode. During the contact of the phases, Ag+ passes into micelles and Na+ passes into emulsion microdroplets through the cation exchange AOTNaOrg + AgNO3Aq = AOTAgOrg + NaNO3Aq. High concentrations of NaNO3 and hydrazine in the microdroplets favor an osmotic outflow of water from the micelles, which reduces their polar cavities to ∼2 nm. As a result, silver ions are contained in the micelles, and the reducing agent is present mostly in emulsion microdroplets. The reagents interact in the polar cavities of micelles to form ∼7 nm Ag nanoparticles. The produced nanoparticles are positively charged, which permitted their electrophoretic concentration to obtain liquid concentrates (up to 30% Ag) and a solid Ag–AOT composite (up to 75% Ag). Their treatment at 250 °C leads to the formation of conductive films (180 mOhm per square). The developed technique makes it possible to increase the productivity of the process by ∼30 times and opens up new avenues of practical application for the well-studied microemulsion synthesis.
中文翻译:

AOT稳定的银纳米粒子有机溶胶的合成与浓缩:乳液与微乳液
在这项工作中,我们试图结合微乳液和乳液合成的优势,以获得稳定的Ag纳米颗粒浓缩有机溶胶,这是很有前途的液相材料。将起始试剂以动态反向乳液模式连续地引入到双-(2-乙基己基)磺基琥珀酸钠(AOT)在正癸烷中的胶束溶液中。在相接触期间,Ag +通过阳离子交换AOTNa Org + AgNO 3 Aq = AOTAg Org + NaNO 3 Aq进入胶束,Na +进入乳液微滴。高浓度的NaNO 3微滴中的肼和水有利于水从胶束中渗透出来,从而将其极性腔减小到约2 nm。结果,胶束中包含银离子,并且还原剂主要存在于乳液微滴中。试剂在胶束的极性腔中相互作用,形成约7 nm的Ag纳米颗粒。产生的纳米粒子带正电,从而允许其电泳浓缩以获得液体浓缩物(最高30%Ag)和固体Ag-AOT复合物(最高75%Ag)。它们在250°C的温度下处理导致形成导电膜(每平方180 mOhm)。所开发的技术可以使该方法的生产率提高约30倍,并为经过深入研究的微乳液合成开辟了实际应用的新途径。
更新日期:2018-02-12
中文翻译:

AOT稳定的银纳米粒子有机溶胶的合成与浓缩:乳液与微乳液
在这项工作中,我们试图结合微乳液和乳液合成的优势,以获得稳定的Ag纳米颗粒浓缩有机溶胶,这是很有前途的液相材料。将起始试剂以动态反向乳液模式连续地引入到双-(2-乙基己基)磺基琥珀酸钠(AOT)在正癸烷中的胶束溶液中。在相接触期间,Ag +通过阳离子交换AOTNa Org + AgNO 3 Aq = AOTAg Org + NaNO 3 Aq进入胶束,Na +进入乳液微滴。高浓度的NaNO 3微滴中的肼和水有利于水从胶束中渗透出来,从而将其极性腔减小到约2 nm。结果,胶束中包含银离子,并且还原剂主要存在于乳液微滴中。试剂在胶束的极性腔中相互作用,形成约7 nm的Ag纳米颗粒。产生的纳米粒子带正电,从而允许其电泳浓缩以获得液体浓缩物(最高30%Ag)和固体Ag-AOT复合物(最高75%Ag)。它们在250°C的温度下处理导致形成导电膜(每平方180 mOhm)。所开发的技术可以使该方法的生产率提高约30倍,并为经过深入研究的微乳液合成开辟了实际应用的新途径。







































 京公网安备 11010802027423号
京公网安备 11010802027423号