当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One-Step Route Synthesis of Siliceous Six-Line Ferrihydrite: Implication for the Formation of Natural Ferrihydrite
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2019-02-01 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00179 Seungyeol Lee 1 , Huifang Xu 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2019-02-01 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00179 Seungyeol Lee 1 , Huifang Xu 1
Affiliation
|
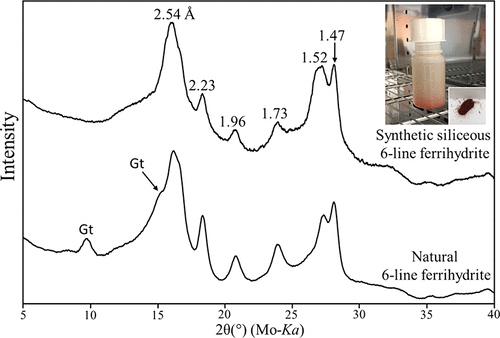
This paper presents a one-step route to the production of siliceous six-line ferrihydrite (6LFh) by oxidizing the aqueous Fe2+–silicate metal complex in solution. Synthetic 6LFh is analogous to naturally occurring 6LFh based on X-ray diffraction and transmission electron microscopy results. We also synthesized siliceous ferrihydrites in different conditions to understand the formation mechanism. The experimental results show the continuous series of siliceous ferrihydrite between two and six lines by increasing the Fe/Si molar ratio, temperature, and reaction time. The results suggest that the presence of Si in solution is the main factor of ferrihydrite formation. Without dissolved silica, only crystalline iron oxyhydroxides, such as goethite and akaganéite, form. Silica inhibits the transformation of ferrihydrite to the more crystalline iron oxyhydroxides. The synthetic experiments can explain the ferrihydrite formation at the Chocolate Pots hot spring in Yellowstone National Park, which represents similar ferrous iron concentration and circumneutral pH thermal spring environments. In comparison to Si-free ferrihydrite, synthetic siliceous ferrihydrite can provide the actual physical and chemical properties of ferrihydrite in natural environments because natural ferrihydrite always contains Si. The new synthesis method will help us better understand the ferrihydrite formations in various geological environments.
中文翻译:

硅质六线水铁矿的一步法合成:对天然水铁矿形成的启示
本文提出了一种通过氧化Fe 2+水溶液来生产硅质六线铁水合物(6LFh)的一步式方法溶液中的–硅酸盐金属络合物。基于X射线衍射和透射电子显微镜结果,合成的6LFh类似于天然存在的6LFh。我们还合成了不同条件下的硅质水铁矿,以了解其形成机理。实验结果表明,通过增加Fe / Si摩尔比,温度和反应时间,硅质铁水合物在2条和6条线之间连续排列。结果表明,溶液中Si的存在是形成水铁矿的主要因素。没有溶解的二氧化硅,仅形成针状铁矿和高锰铁矿的结晶羟基氧化铁。二氧化硅可抑制亚铁酸盐转变为结晶性更高的羟基氧化铁。合成实验可以解释黄石国家公园巧克力罐温泉中的水铁矿形成,这代表了类似的亚铁浓度和周围pH的温泉环境。与不含硅的水铁矿相比,合成的硅质水铁矿可以提供自然环境中的水铁矿的实际物理和化学特性,因为天然水铁矿中总是含有硅。新的合成方法将帮助我们更好地了解各种地质环境中的水铁矿形成。合成的硅质水铁矿可以提供自然环境中水铁矿的实际物理和化学性质,因为天然水铁矿中总是含有硅。新的合成方法将帮助我们更好地了解各种地质环境中的水铁矿形成。合成的硅质水铁矿可以提供自然环境中水铁矿的实际物理和化学特性,因为天然水铁矿中总是含有硅。新的合成方法将帮助我们更好地了解各种地质环境中的水铁矿形成。
更新日期:2019-02-01
中文翻译:

硅质六线水铁矿的一步法合成:对天然水铁矿形成的启示
本文提出了一种通过氧化Fe 2+水溶液来生产硅质六线铁水合物(6LFh)的一步式方法溶液中的–硅酸盐金属络合物。基于X射线衍射和透射电子显微镜结果,合成的6LFh类似于天然存在的6LFh。我们还合成了不同条件下的硅质水铁矿,以了解其形成机理。实验结果表明,通过增加Fe / Si摩尔比,温度和反应时间,硅质铁水合物在2条和6条线之间连续排列。结果表明,溶液中Si的存在是形成水铁矿的主要因素。没有溶解的二氧化硅,仅形成针状铁矿和高锰铁矿的结晶羟基氧化铁。二氧化硅可抑制亚铁酸盐转变为结晶性更高的羟基氧化铁。合成实验可以解释黄石国家公园巧克力罐温泉中的水铁矿形成,这代表了类似的亚铁浓度和周围pH的温泉环境。与不含硅的水铁矿相比,合成的硅质水铁矿可以提供自然环境中的水铁矿的实际物理和化学特性,因为天然水铁矿中总是含有硅。新的合成方法将帮助我们更好地了解各种地质环境中的水铁矿形成。合成的硅质水铁矿可以提供自然环境中水铁矿的实际物理和化学性质,因为天然水铁矿中总是含有硅。新的合成方法将帮助我们更好地了解各种地质环境中的水铁矿形成。合成的硅质水铁矿可以提供自然环境中水铁矿的实际物理和化学特性,因为天然水铁矿中总是含有硅。新的合成方法将帮助我们更好地了解各种地质环境中的水铁矿形成。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号