当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantiopure Methyl- and Phenyllithium: Mixed (Carb-)Anionic Anisyl Fencholate-Aggregates
Organometallics ( IF 2.5 ) Pub Date : 2019-02-05 , DOI: 10.1021/acs.organomet.8b00724 Vanessa Grote 1 , Jörg-Martin Neudörfl 1 , Bernd Goldfuss 1
Organometallics ( IF 2.5 ) Pub Date : 2019-02-05 , DOI: 10.1021/acs.organomet.8b00724 Vanessa Grote 1 , Jörg-Martin Neudörfl 1 , Bernd Goldfuss 1
Affiliation
|
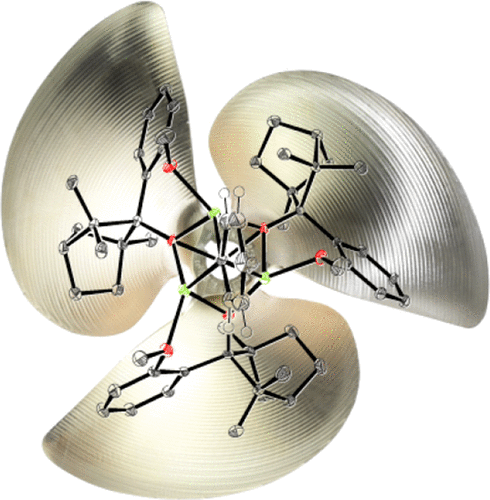
Methyl- and phenyllithium aggregates with enantiopure anisyl fencholate units form after reaction of organolithium reagent with (+)-anisyl fenchol in hydrocarbon and some ethereal solvents. These carbanionic aggregates are characterized by X-ray crystal analyses and exhibit both 3:1 stoichiometry and distorted cubic Li4O3C1 cores, in which three lithium ions coordinate the carbanion (i.e., methylide or phenylide). These three lithium ions define a Lewis acidic surface (Li3), binding the carbanion and expanding with the steric demand of the carbanion (i.e., from Me: 2.62 Å2, over n-Bu: 2.65 Å2 (previous work) to Ph: 2.79 Å2). Methylation and phenylation reactions of various prochiral aldehydes employing these methyllithium and phenyllithium aggregates yield alcohols with up to 44% ee. To rationalize the formation of the mixed (carb-)anionic aggregates, aggregate formation energies, describing co-condensations of RLi (R = Me, Ph, n-Bu) and lithium fencholates, are computed for the 3:1 and 2:2 stoichiometries. These computed aggregate formation energies point to preferences for 3:1 over 2:2 aggregates, as it is also apparent from experimental aggregate formations, confirmed by X-ray crystal analyses. In close analogy to the X-ray crystal structures, the computed Li3 surfaces increase with increasing steric demand of the carbanions. The chiral, mixed (carb-)anionic RLi-fencholate aggregates hence adapt to different carbanion sized and arise not only with small (Me) or primary carbanions (n-Bu) but even with the larger secondary phenyl anion.
中文翻译:

对映体纯的甲基和苯基锂:混合的(碳-)阴离子茴香酸苯甲酸酯-聚集体
在有机锂试剂与(+)-茴香基苯酚在烃和某些醚溶剂中反应后,形成具有对映体纯茴香酸苯酚酯单元的甲基和苯基锂聚集体。这些碳负离子聚集体通过X射线晶体分析进行表征,并且显示出3:1的化学计量比和扭曲的立方Li 4 O 3 C 1核,其中三个锂离子配位碳负离子(即,亚甲基或亚苯基)。这三个锂离子限定路易斯酸性表面(栗3),结合碳负离子,并用负碳离子(的立体需求扩大即,从我:2.62埃2,经Ñ -Bu:2.65 2(先前的工作)至pH :2.79埃2)。使用这些甲基锂和苯基锂聚集体的各种前手性醛的甲基化和苯基化反应生成的ee含量高达44%。为了合理化混合的(碳-)阴离子聚集体的形成,对于3:1和2:2,计算了描述RLi(R = Me,Ph,n -Bu)和苯酚锂的共缩合的聚集体形成能。化学计量。这些计算出的聚集体形成能表明,相对于2:2聚集体而言,它们具有3:1的偏好性,这也是从X射线晶体分析证实的实验聚集体形成中显而易见的。与X射线晶体结构非常相似,计算出的Li 3随着碳的空间需求增加,表面增加。因此,手性的,混合的(碳-)阴离子的RLi-苯酚酸盐聚集体适应于不同的碳负离子尺寸,并且不仅会与较小的(Me)或伯碳负离子(n- Bu)一起出现,甚至会与较大的仲苯基阴离子一起出现。
更新日期:2019-02-06
中文翻译:

对映体纯的甲基和苯基锂:混合的(碳-)阴离子茴香酸苯甲酸酯-聚集体
在有机锂试剂与(+)-茴香基苯酚在烃和某些醚溶剂中反应后,形成具有对映体纯茴香酸苯酚酯单元的甲基和苯基锂聚集体。这些碳负离子聚集体通过X射线晶体分析进行表征,并且显示出3:1的化学计量比和扭曲的立方Li 4 O 3 C 1核,其中三个锂离子配位碳负离子(即,亚甲基或亚苯基)。这三个锂离子限定路易斯酸性表面(栗3),结合碳负离子,并用负碳离子(的立体需求扩大即,从我:2.62埃2,经Ñ -Bu:2.65 2(先前的工作)至pH :2.79埃2)。使用这些甲基锂和苯基锂聚集体的各种前手性醛的甲基化和苯基化反应生成的ee含量高达44%。为了合理化混合的(碳-)阴离子聚集体的形成,对于3:1和2:2,计算了描述RLi(R = Me,Ph,n -Bu)和苯酚锂的共缩合的聚集体形成能。化学计量。这些计算出的聚集体形成能表明,相对于2:2聚集体而言,它们具有3:1的偏好性,这也是从X射线晶体分析证实的实验聚集体形成中显而易见的。与X射线晶体结构非常相似,计算出的Li 3随着碳的空间需求增加,表面增加。因此,手性的,混合的(碳-)阴离子的RLi-苯酚酸盐聚集体适应于不同的碳负离子尺寸,并且不仅会与较小的(Me)或伯碳负离子(n- Bu)一起出现,甚至会与较大的仲苯基阴离子一起出现。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号