当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of Nitric Oxide Reduction by Hydrogen on Ni(110) and Ir/Ni(110): First Principles and Microkinetic Modeling
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-02-15 , DOI: 10.1021/acs.jpcc.8b10806 Hong Wen 1 , Li-yuan Huai 1 , Xin Jin 1 , Jing-yao Liu 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-02-15 , DOI: 10.1021/acs.jpcc.8b10806 Hong Wen 1 , Li-yuan Huai 1 , Xin Jin 1 , Jing-yao Liu 1
Affiliation
|
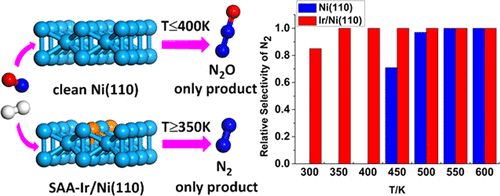
The nitric oxide (NO) reduction by H2 on the pure Ni(110) and single-atom Ir-doped Ni(110) (Ir/Ni(110)) surfaces are investigated by density functional theory calculations coupled with microkinetic modeling. The stability of the single-atom alloy (SAA) Ir/Ni(110) in vacuum and under real conditions is considered. The results show that NO dissociation is very facile on the clean and H-predosed Ni(110) surface, and the dissociated N atoms is more likely to couple with the surface-adsorbed NO to produce N2O than with the N atom to form N2. However, the energy barrier for N2 formation is largely lowered by the doped Ir, whereas that for N2O formation is greatly increased. Thus, N2 is energetically preferentially formed on the Ir/Ni(110) surface. Microkinetic calculations further demonstrate that under ultrahigh-vacuum conditions, the selectivity toward N2O is 100% below 420 K and that toward N2 is over 80% above 460 K, in line with the experimental observation. In contrast, Ir/Ni(110) exhibits markedly improved selectivity toward N2 in a wide temperature range (>90% at 320 K and 100% at T ≥ 340 K). The present work shows that the SAA Ir/Ni(110) catalyst is very effective in promoting the reduction of NO into N2 while largely suppressing N2O formation.
中文翻译:

氢在Ni(110)和Ir / Ni(110)上还原氢一氧化氮的机理:第一性原理和微动力学模型
通过密度泛函理论计算和微动力学模型研究了纯Ni(110)和单原子掺Ir(Ni / 110)(Ir / Ni(110))表面上H 2对一氧化氮(NO)的还原作用。考虑了单原子合金(SAA)Ir / Ni(110)在真空和实际条件下的稳定性。结果表明,在干净且H掺杂的Ni(110)表面上,NO的解离非常容易,并且解离的N原子更容易与表面吸附的NO结合生成N 2 O,而不是与N原子形成。 N 2。然而,通过掺杂的Ir,N 2形成的能垒大大降低,而N 2 O形成的能垒大大提高。因此,N 2能量优先地在Ir / Ni(110)表面上形成。Microkinetic计算进一步证明,超高真空条件下,向N选择性2 O为100%以下420 K和朝向Ñ 2超过80%以上460 K,与实验观察一致。与此相反,IR /镍(110)呈现朝N显着改善的选择性2在宽温度范围内(>在约32 90%,并在100%Ť ≥340 K)。目前的工作表明,SAA Ir / Ni(110)催化剂在促进NO还原成N 2的同时非常抑制N 2 O的形成方面非常有效。
更新日期:2019-02-19
中文翻译:

氢在Ni(110)和Ir / Ni(110)上还原氢一氧化氮的机理:第一性原理和微动力学模型
通过密度泛函理论计算和微动力学模型研究了纯Ni(110)和单原子掺Ir(Ni / 110)(Ir / Ni(110))表面上H 2对一氧化氮(NO)的还原作用。考虑了单原子合金(SAA)Ir / Ni(110)在真空和实际条件下的稳定性。结果表明,在干净且H掺杂的Ni(110)表面上,NO的解离非常容易,并且解离的N原子更容易与表面吸附的NO结合生成N 2 O,而不是与N原子形成。 N 2。然而,通过掺杂的Ir,N 2形成的能垒大大降低,而N 2 O形成的能垒大大提高。因此,N 2能量优先地在Ir / Ni(110)表面上形成。Microkinetic计算进一步证明,超高真空条件下,向N选择性2 O为100%以下420 K和朝向Ñ 2超过80%以上460 K,与实验观察一致。与此相反,IR /镍(110)呈现朝N显着改善的选择性2在宽温度范围内(>在约32 90%,并在100%Ť ≥340 K)。目前的工作表明,SAA Ir / Ni(110)催化剂在促进NO还原成N 2的同时非常抑制N 2 O的形成方面非常有效。











































 京公网安备 11010802027423号
京公网安备 11010802027423号