当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparing Rate and Mechanism of Ethane Hydrogenolysis on Transition-Metal Catalysts
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-02-21 , DOI: 10.1021/acs.jpcc.8b11070 Abdulrahman Almithn 1 , David Hibbitts 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-02-21 , DOI: 10.1021/acs.jpcc.8b11070 Abdulrahman Almithn 1 , David Hibbitts 1
Affiliation
|
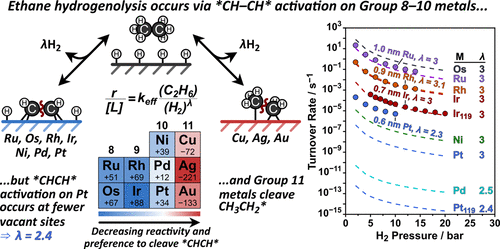
The effects of metal catalyst identity on the ethane hydrogenolysis rates and mechanism were examined using density functional theory (DFT) for Group 8–11 metals (Ru, Os, Rh, Ir, Ni, Pd, Pt, Cu, Ag, and Au). Previously measured turnover rates on Ru, Rh, and Ir clusters show H2-pressure dependence of [H2]–3, consistent with C–C bond activation in *CHCH* intermediates in reactions that require two H* (chemisorbed H) to desorb from the H*-covered surfaces that prevail at these hydrogenolysis conditions. Previous DFT calculations on Ir catalysts have shown that C–C bonds in alkanes are weakened by forming C–metal bonds through quasi-equilibrated dehydrogenation steps during ethane hydrogenolysis, and these steps form *CHCH* intermediates which undergo a kinetically relevant C–C bond cleavage step. Here, the DFT-calculated free-energy barriers show that *CH–CH* bond activation is also more favorable than all C–C bond activations in other intermediates on Group 8–10 metals by >34 kJ mol–1 with the exception of Pd, where *CHCH* and CH3CH* activate with similar activation free energies (242 and 253 kJ mol–1, respectively, 593 K). The relative free-energy barriers between *CH–CH* bond cleavage and C–C bond cleavage in more saturated intermediates decrease as one moves from left to right in the periodic table until *CH3–CH2* bond cleavage becomes more favorable on Group 11 coinage metals (Cu, Ag, and Au). Such predicted trends are consistent with the measured turnover rates that decrease as Ru > Rh > Ir > Pt and show H2-pressure dependence of ∼[H2]–3 (λ = 3) for Ru, Rh, and Ir clusters and [H2]–2.3 (λ = 2.3) for Pt clusters. The decrease in the measured λ value for Pt, however, is caused by a decrease in the number of desorbed H* atoms from the surface (γ = 0–1) rather than a change in the mechanism as shown here using a H*-covered Pt119 half-particle model. The lower H*-coverage on Pt compared to other metals and the lateral relaxation of the adlayer in curved nanoparticle models, as reported previously, allow *CH–CH* bond cleavage to occur at a lower number of vacant sites on Pt.
中文翻译:

过渡金属催化剂上乙烷氢解反应速率及机理的比较
使用密度泛函理论(DFT)对第8-11组金属(Ru,Os,Rh,Ir,Ni,Pd,Pt,Cu,Ag和Au)进行了金属催化剂身份对乙烷氢解速率和机理的影响研究。 。先前测量的Ru,Rh和Ir簇上的周转率显示[H 2 ] –3的H 2-压力依赖性,与* CHCH *中间体中C–C键的活化作用相一致,该反应需要两个H *(化学吸附的H)从在这些氢解条件下普遍存在的被H *覆盖的表面解吸。先前对Ir催化剂的DFT计算表明,乙烷加氢分解过程中通过准平衡的脱氢步骤形成C-金属键,从而削弱了烷烃中的C-C键,这些步骤形成了* CHCH *中间体,这些中间体经历了动力学相关的C-C键裂解步骤。在这里,DFT计算的自由能垒表明,* CH-CH *键活化比第8-10组金属上其他中间体中的所有C-C键活化都要好34 kJ mol –1,比Pd,其中* CHCH *和CH 3CH *激活具有相似活化自由能(242和253千焦耳摩尔-1,分别,593 K)。在更饱和的中间体中,* CH-CH *键断裂和CC键断裂之间的相对自由能垒随着周期表中从左向右移动直到* CH 3 -CH 2 *键断裂变得更有利而降低。第11组造币金属(Cu,Ag和Au)。这样的预测趋势与所测得的周转率一致,随着Ru> Rh> Ir> Ir> Pt的降低,Ru,Rh和Ir簇和[ [H 2 ] –3(λ= 3)的H 2压力依赖性。H 2 ] –2.3(λ= 2.3)对于Pt簇。但是,测得的Pt的λ值降低是由于从表面解吸的H *原子数量减少(γ= 0-1),而不是此处所示的使用H *-的机理改变。涵盖了Pt 119半粒子模型。如前所述,与其他金属相比,Pt上的H *覆盖率较低,并且吸附层在弯曲纳米颗粒模型中的横向弛豫,使得* CH-CH *键的裂解发生在Pt上的空位较少。
更新日期:2019-02-21
中文翻译:

过渡金属催化剂上乙烷氢解反应速率及机理的比较
使用密度泛函理论(DFT)对第8-11组金属(Ru,Os,Rh,Ir,Ni,Pd,Pt,Cu,Ag和Au)进行了金属催化剂身份对乙烷氢解速率和机理的影响研究。 。先前测量的Ru,Rh和Ir簇上的周转率显示[H 2 ] –3的H 2-压力依赖性,与* CHCH *中间体中C–C键的活化作用相一致,该反应需要两个H *(化学吸附的H)从在这些氢解条件下普遍存在的被H *覆盖的表面解吸。先前对Ir催化剂的DFT计算表明,乙烷加氢分解过程中通过准平衡的脱氢步骤形成C-金属键,从而削弱了烷烃中的C-C键,这些步骤形成了* CHCH *中间体,这些中间体经历了动力学相关的C-C键裂解步骤。在这里,DFT计算的自由能垒表明,* CH-CH *键活化比第8-10组金属上其他中间体中的所有C-C键活化都要好34 kJ mol –1,比Pd,其中* CHCH *和CH 3CH *激活具有相似活化自由能(242和253千焦耳摩尔-1,分别,593 K)。在更饱和的中间体中,* CH-CH *键断裂和CC键断裂之间的相对自由能垒随着周期表中从左向右移动直到* CH 3 -CH 2 *键断裂变得更有利而降低。第11组造币金属(Cu,Ag和Au)。这样的预测趋势与所测得的周转率一致,随着Ru> Rh> Ir> Ir> Pt的降低,Ru,Rh和Ir簇和[ [H 2 ] –3(λ= 3)的H 2压力依赖性。H 2 ] –2.3(λ= 2.3)对于Pt簇。但是,测得的Pt的λ值降低是由于从表面解吸的H *原子数量减少(γ= 0-1),而不是此处所示的使用H *-的机理改变。涵盖了Pt 119半粒子模型。如前所述,与其他金属相比,Pt上的H *覆盖率较低,并且吸附层在弯曲纳米颗粒模型中的横向弛豫,使得* CH-CH *键的裂解发生在Pt上的空位较少。

































 京公网安备 11010802027423号
京公网安备 11010802027423号