当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unpicking the Cause of Stereoselectivity in Actinorhodin Ketoreductase Variants with Atomistic Simulations
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-01-31 00:00:00 , DOI: 10.1021/acscatal.8b04846 Stefano A. Serapian 1 , Marc W. van der Kamp 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-01-31 00:00:00 , DOI: 10.1021/acscatal.8b04846 Stefano A. Serapian 1 , Marc W. van der Kamp 1, 2
Affiliation
|
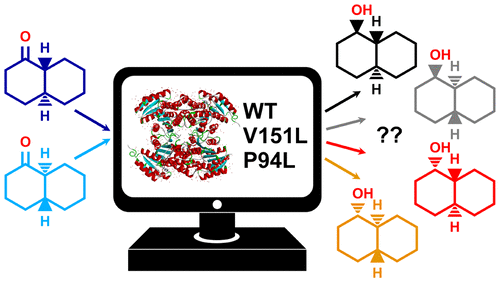
Ketoreductase enzymes (KRs) with a high degree of regio- and stereoselectivity are useful biocatalysts for the production of small, specific chiral alcohols from achiral ketones. Actinorhodin KR (actKR), part of a type II polyketide synthase involved in the biosynthesis of the antibiotic actinorhodin, can also turn over small ketones. In vitro studies assessing stereocontrol in actKR have found that, in the “reverse” direction, the wild-type (WT) enzyme’s mild preference for S-α-tetralol is enhanced by certain mutations (e.g., P94L) and entirely reversed by others (e.g., V151L) in favor of R-α-tetralol. Here, we employ computationally cost-effective atomistic simulations to rationalize these trends in WT, P94L, and V151L actKR using trans-1-decalone (1) as the model substrate. Three potential factors (FI–FIII) are investigated: frequency of pro-R vs pro-S reactive poses (FI) is assessed with classical molecular dynamics (MD), binding affinity of pro-R vs pro-S orientations (FII) is compared using the binding free energy method MM/PBSA, and differences in reaction barriers toward trans-1-decalol (FIII) are assessed by hybrid semiempirical quantum/classical (QM/MM) MD simulations with umbrella sampling, benchmarked with density functional theory. No single factor is found to dominate stereocontrol: FI largely determines the selectivity of V151L actKR, whereas FIII is more dominant in the case of P94L. It is also found that formation of S-trans-1-decalol or R-trans-1-decalol mainly arises from the reduction of the trans-1-decalone enantiomers (4aS,8aR)-1 or (4aR,8aS)-1, respectively. Our work highlights the complexity of enzyme stereoselectivity as well as the usefulness of atomistic simulations to aid the design of stereoselective biocatalysts.
中文翻译:

用原子模拟找出放线菌丝氨酸酮还原酶变体中立体选择性的原因
具有高度的区域和立体选择性的酮还原酶(KR)是有用的生物催化剂,用于从非手性酮生产小的特定手性醇。放线菌素KR(act KR)是II型聚酮化合物合酶的一部分,它参与抗生素放线菌素的生物合成,也可以上交小酮。评估动作KR中立体控制的体外研究发现,在“反向”方向上,某些突变(例如P94L)增强了野生型(WT)酶对S-α-四氢萘酚的轻度偏爱,而其他突变则完全逆转了(例如V151L)支持R-α-四氢萘酚。在这里,我们采用具有计算成本效益的原子模拟来合理化WT,P94L和V151L中的这些趋势作用使用KR反式1萘烷酮(1)作为模型基板。研究了三个潜在因素(FI – FIII):用经典分子动力学(MD)评估pro-R对pro-S的反应姿势(FI)的频率,pro-R对pro-S取向的结合亲和力(FII)为使用结合自由能方法MM / PBSA进行比较,以及对反式1-癸醇(FIII)是通过混合半经验量子/经典(QM / MM)MD模拟和伞形采样(以密度泛函理论为基准)进行评估的。没有发现一个因素可以控制立体声控制:FI在很大程度上决定了V151L act KR的选择性,而在P94L的情况下,FIII更具优势。它也发现,形成的S-反式-1- decalol或R-反式-1- decalol主要来自于还原反式-1-萘烷酮对映异构体(4AS,8AR) - 1或(4AR,8AS) - 1, 分别。我们的工作突出了酶立体选择性的复杂性,以及原子模拟有助于立体选择性生物催化剂设计的有用性。
更新日期:2019-01-31
中文翻译:

用原子模拟找出放线菌丝氨酸酮还原酶变体中立体选择性的原因
具有高度的区域和立体选择性的酮还原酶(KR)是有用的生物催化剂,用于从非手性酮生产小的特定手性醇。放线菌素KR(act KR)是II型聚酮化合物合酶的一部分,它参与抗生素放线菌素的生物合成,也可以上交小酮。评估动作KR中立体控制的体外研究发现,在“反向”方向上,某些突变(例如P94L)增强了野生型(WT)酶对S-α-四氢萘酚的轻度偏爱,而其他突变则完全逆转了(例如V151L)支持R-α-四氢萘酚。在这里,我们采用具有计算成本效益的原子模拟来合理化WT,P94L和V151L中的这些趋势作用使用KR反式1萘烷酮(1)作为模型基板。研究了三个潜在因素(FI – FIII):用经典分子动力学(MD)评估pro-R对pro-S的反应姿势(FI)的频率,pro-R对pro-S取向的结合亲和力(FII)为使用结合自由能方法MM / PBSA进行比较,以及对反式1-癸醇(FIII)是通过混合半经验量子/经典(QM / MM)MD模拟和伞形采样(以密度泛函理论为基准)进行评估的。没有发现一个因素可以控制立体声控制:FI在很大程度上决定了V151L act KR的选择性,而在P94L的情况下,FIII更具优势。它也发现,形成的S-反式-1- decalol或R-反式-1- decalol主要来自于还原反式-1-萘烷酮对映异构体(4AS,8AR) - 1或(4AR,8AS) - 1, 分别。我们的工作突出了酶立体选择性的复杂性,以及原子模拟有助于立体选择性生物催化剂设计的有用性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号