当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Unveiling of C═C Double-Bond Rotation and Origins of Regioselectivity and Product E/Z Selectivity of Pd-Catalyzed Olefinic C–H Functionalization of (E)-N-Methoxy Cinnamamide
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-02-05 00:00:00 , DOI: 10.1021/acs.joc.7b03007 Lingjun Liu 1 , Guojing Pei 1 , Peng Liu 1 , Baoping Ling 1 , Yuxia Liu 1 , Siwei Bi 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-02-05 00:00:00 , DOI: 10.1021/acs.joc.7b03007 Lingjun Liu 1 , Guojing Pei 1 , Peng Liu 1 , Baoping Ling 1 , Yuxia Liu 1 , Siwei Bi 1
Affiliation
|
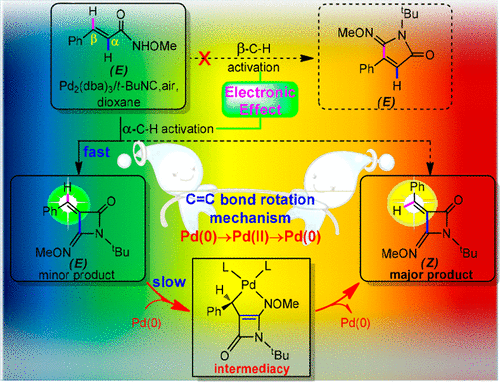
Density functional theory (DFT) calculations have been performed to study the Pd-catalyzed C—H functionalization of (E)-N-methoxy cinnamamide (E1), which selectively provides the α-C—H activation products (EP as minor product and its C═C rotation isomer ZP′ as major product). Three crucial issues are solved: (i) The detailed mechanism leading to ZP′ is one issue. The computational analyses of the mechanisms proposed in previously experimental and theoretical literature do not seem to be consistent with the experimental findings due to the high barriers involved. Alternatively, we present a novel oxidation/reduction-promoted mechanism featuring the Pd(0) → Pd(II) → Pd(0) transformation. The newly proposed mechanism involves the initial coordination of the active catalyst PdL2 (L = t-BuCN) with the C═C bond in EP, followed by the oxidative cyclization/reductive decyclization-assisted C═C double-bond rotation processes resulting in ZP′ and regeneration of PdL2. (ii) The origin of the product E/Z selectivity is the second issue. On the basis of the calculated results, it is found that, at the initial stage of the reaction, EP is certainly completely generated, while no ZP′ formation occurred. Once E1 is used up, EP immediately acts as the partner of the new catalytic cycle and sluggishly evolves into ZP′. A small amount of generated ZP′ would reversibly transform to EP due to the higher barrier involved. (iii) The intrinsic reasons for the regioselectivity are the third issue. The calculated results indicate that the regioselectivity for α-C—H activation is mainly attributed to the stronger electrostatic attraction between the α-C and the metal center.
中文翻译:

C═C双键旋转的机理揭示以及(E)-N-甲氧基肉桂酰胺Pd催化的烯烃C–H官能化的区域选择性和产物E / Z选择性的起源
已经进行了密度泛函理论(DFT)计算,以研究Pd催化的(E)-N-甲氧基肉桂酰胺(E 1)的C-H官能化,该选择性地提供了α-CHH活化产物(E P为次要产物及其C═C旋转异构体Z P'为主要产物)。解决了三个关键问题:(i)导致Z P '的详细机制是一个问题。由于涉及较高的障碍,对先前实验和理论文献中提出的机理进行的计算分析似乎与实验结果不一致。或者,我们提出了以Pd(0)→Pd(II)→Pd(0)转变为特征的新型氧化/还原促进机制。新提出的机制涉及活性催化剂PdL 2(L = t -BuCN)与E P中的C═C键的初始配位,然后进行氧化环化/还原脱环辅助的C═C双键旋转过程在ž P'和PDL的再生2。(ii)产品的原产地E /Z选择性是第二个问题。根据计算结果,发现在反应的初始阶段,肯定会完全生成E P,而不会发生Z P'的形成。一旦E 1用完,E P立即充当新催化循环的伙伴,并缓慢地演变为Z P'。少量生成的Z P'将可逆地转换为E P由于涉及更高的障碍。(iii)区域选择性的内在原因是第三个问题。计算结果表明,α-CH活化的区域选择性主要归因于α-C与金属中心之间较强的静电吸引。
更新日期:2018-02-05
中文翻译:

C═C双键旋转的机理揭示以及(E)-N-甲氧基肉桂酰胺Pd催化的烯烃C–H官能化的区域选择性和产物E / Z选择性的起源
已经进行了密度泛函理论(DFT)计算,以研究Pd催化的(E)-N-甲氧基肉桂酰胺(E 1)的C-H官能化,该选择性地提供了α-CHH活化产物(E P为次要产物及其C═C旋转异构体Z P'为主要产物)。解决了三个关键问题:(i)导致Z P '的详细机制是一个问题。由于涉及较高的障碍,对先前实验和理论文献中提出的机理进行的计算分析似乎与实验结果不一致。或者,我们提出了以Pd(0)→Pd(II)→Pd(0)转变为特征的新型氧化/还原促进机制。新提出的机制涉及活性催化剂PdL 2(L = t -BuCN)与E P中的C═C键的初始配位,然后进行氧化环化/还原脱环辅助的C═C双键旋转过程在ž P'和PDL的再生2。(ii)产品的原产地E /Z选择性是第二个问题。根据计算结果,发现在反应的初始阶段,肯定会完全生成E P,而不会发生Z P'的形成。一旦E 1用完,E P立即充当新催化循环的伙伴,并缓慢地演变为Z P'。少量生成的Z P'将可逆地转换为E P由于涉及更高的障碍。(iii)区域选择性的内在原因是第三个问题。计算结果表明,α-CH活化的区域选择性主要归因于α-C与金属中心之间较强的静电吸引。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号