当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, Neuroprotective and Antibacterial Activities of 1,2,4‐Triazolo[3,4‐b]1,3,4‐thiadiazole Linked Thieno[2,3‐d]pyrimidine Derivatives and In Silico Docking Studies
ChemistrySelect ( IF 1.9 ) Pub Date : 2019-02-01 , DOI: 10.1002/slct.201803917 Triloknadh Settypalli 1 , Venkata Rao Chunduri 1 , Nagaraju Kerru 1 , Hari Krishna Nallapaneni 1 , Venkata Ramaiah Chintha 2 , Trinath Daggupati 2 , Suneetha Yeguvapalli 2 , Rajendra Wudayagiri 2
ChemistrySelect ( IF 1.9 ) Pub Date : 2019-02-01 , DOI: 10.1002/slct.201803917 Triloknadh Settypalli 1 , Venkata Rao Chunduri 1 , Nagaraju Kerru 1 , Hari Krishna Nallapaneni 1 , Venkata Ramaiah Chintha 2 , Trinath Daggupati 2 , Suneetha Yeguvapalli 2 , Rajendra Wudayagiri 2
Affiliation
|
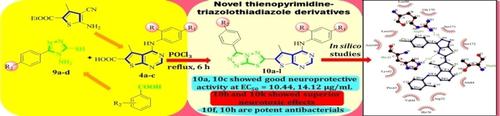
In pursuit of neuroprotective and antimicrobial agents, a series of 1,2,4‐triazolo[3,4‐b]1,3,4‐thiadiazole incorporated thieno[2,3‐d]pyrimidine derivatives 10 a‐l has been designed, synthesized. The final target compounds were screened for neuroprotective, neurotoxic and antibacterial activities. The compounds derived from 4‐methylphenyl (10 a) and 4‐nitrophenyl (10 c) have showed good neuroprotective activity against H2O2 induced PC12 cell death at respective EC50 values of 10.44, 14.12 μg/mL. However 10 b and 10 k showed superior neurotoxic effects than rest of the compounds with respective CC50 values of 100.16, 120 μg/mL. Potent antibacterial activity was shown by 10 f (R=‐Me, R2=‐OMe), 10 h (R=‐Me, R2=‐Cl) against the four bacterial pathogens such as S.aureus, B.subtilis, E.coli and P.aeruginosa at low minimum inhibitory concentration (MIC) range. Further, in silico docking studies were performed for all the synthesized compounds with C(30) carotenoid dehydrosqualene synthase, Gyrase A and LpxC bacterial proteins. Interestingly, 10 f, 10 h showed good binding affinities with target proteins and these results are in good compliance with the in vitro activity profile data.
中文翻译:

1,2,4-三唑并[3,4-b] 1,3,4-噻二唑连接的噻吩并[2,3-d]嘧啶衍生物的设计,合成,神经保护和抗菌活性以及计算机对接研究
在追求神经保护和抗微生物剂的,一系列的1,2,4-三唑并[3,4-B]的1,3,4-噻二唑注册成立噻吩并[2,3-d]嘧啶衍生物10 A-L已被设计,合成。筛选最终目标化合物的神经保护,神经毒性和抗菌活性。从4-甲基苯基(衍生的化合物10)和4-硝基苯基(10℃)都显示出良好的神经保护活性针对ħ 2 ö 2在相应的EC诱导的PC12细胞死亡50 10.44,14.12微克/毫升的值。然而,10 b和10 k的神经毒性作用优于其余分别具有CC 50的化合物值100.16,120μg/ mL。有效的抗菌活性是通过所示10f中(R = -Me,R 2 = -OMe),10 H(R = -Me,R 2 = -Cl)针对四个细菌病原体,如金黄色葡萄球菌,枯草芽孢杆菌,最低抑菌浓度(MIC)较低的大肠杆菌和铜绿假单胞菌。此外,对所有合成的化合物与C(30)类胡萝卜素脱氢角鲨烯合酶,回旋酶A和LpxC细菌蛋白进行了计算机对接研究。有趣的是,在10 f,10 h时,与靶蛋白的结合亲和力很好,并且这些结果与体外实验结果具有很好的一致性。 活动资料数据。
更新日期:2019-02-01
中文翻译:

1,2,4-三唑并[3,4-b] 1,3,4-噻二唑连接的噻吩并[2,3-d]嘧啶衍生物的设计,合成,神经保护和抗菌活性以及计算机对接研究
在追求神经保护和抗微生物剂的,一系列的1,2,4-三唑并[3,4-B]的1,3,4-噻二唑注册成立噻吩并[2,3-d]嘧啶衍生物10 A-L已被设计,合成。筛选最终目标化合物的神经保护,神经毒性和抗菌活性。从4-甲基苯基(衍生的化合物10)和4-硝基苯基(10℃)都显示出良好的神经保护活性针对ħ 2 ö 2在相应的EC诱导的PC12细胞死亡50 10.44,14.12微克/毫升的值。然而,10 b和10 k的神经毒性作用优于其余分别具有CC 50的化合物值100.16,120μg/ mL。有效的抗菌活性是通过所示10f中(R = -Me,R 2 = -OMe),10 H(R = -Me,R 2 = -Cl)针对四个细菌病原体,如金黄色葡萄球菌,枯草芽孢杆菌,最低抑菌浓度(MIC)较低的大肠杆菌和铜绿假单胞菌。此外,对所有合成的化合物与C(30)类胡萝卜素脱氢角鲨烯合酶,回旋酶A和LpxC细菌蛋白进行了计算机对接研究。有趣的是,在10 f,10 h时,与靶蛋白的结合亲和力很好,并且这些结果与体外实验结果具有很好的一致性。 活动资料数据。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号