当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Snapshots of the Hydrolysis of Lithium 4,5-Dicyanoimidazolate–Glyme Solvates. Impact of Water Molecules on Aggregation Processes in Lithium-Ion Battery Electrolytes
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-02-01 00:00:00 , DOI: 10.1021/acs.jpcc.7b11145 Maciej Dranka 1 , Piotr Jankowski 1 , Grażyna Z. Żukowska 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-02-01 00:00:00 , DOI: 10.1021/acs.jpcc.7b11145 Maciej Dranka 1 , Piotr Jankowski 1 , Grażyna Z. Żukowska 1
Affiliation
|
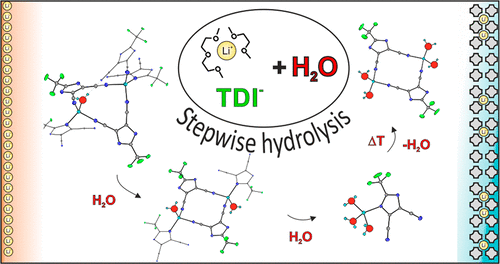
Despite that 4,5-dicyano-2-(trifluoromethyl)imidazole lithium salt (LiTDI) exhibits several interesting features in aprotic solvents such as glymes or carbonate esters, little is known about its structural rearrangement after exposure to water. Since the LiTDI salt has been verified as an effective moisture scavenger able to suppress degradation of the LiPF6-based electrolyte, comprehensive knowledge of coordination modes in the LiTDI–H2O system, as well as information about the structure of formed hydrates, is desirable. In the present study, we report the impact of water on the LiTDI glyme-based electrolytes investigated by means of the single-crystal X-ray diffraction technique and Raman spectroscopy. We have found that the exposure of lithium 4,5-dicyanoimidazolate–glyme solvates to humid air gives rise to the hydrolysis products arising from stepwise addition of water molecules to the lithium coordination sphere. Several structural motifs have been distinguished as preferred coordination modes in the LiTDI–H2O system. A high number of available ether oxygen donor center water molecules cause dissociation of ionic contact pairs and aggregation of cationic species stabilized by crown ethers. Low O:Li molar ratio leads to the formation of LiTDI–glyme–water solvates and LiTDI hydrates. The air-stable LiTDI trihydrate comprises ionic pairs formed by a lithium cation coordinated to an imidazole nitrogen of TDI. A lithium cation coordinated via nitrile groups and bearing water molecules is a basic motif constituting dimeric species of formula [Li(H2O)2TDI]2 which are present in aggregated [Li(H2O)TDI]n chains making up the structure of a monohydrate. The discovered motifs have been proved to occur in both the solid and melted hydrated systems of LiTDI. They will be helpful for conducting molecular dynamic calculations and for obtaining information how to manipulate the structure of a Li+-solvation sheath in both hydrated and liquid aqueous electrolytes based on heterocyclic anions.
中文翻译:

4,5-二氰基咪唑酸锂水解的快照–甘醇二甲醚。水分子对锂离子电池电解质聚集过程的影响
尽管4,5-二氰基-2-(三氟甲基)咪唑锂盐(LiTDI)在非质子传递溶剂(如甘醇二甲酸酯或碳酸酯)中表现出几个有趣的特征,但在暴露于水后其结构重排知之甚少。由于LiTDI盐已被证实是能够抑制基于LiPF 6的电解质降解的有效水分清除剂,因此需要全面了解LiTDI–H 2中的配位模式理想的是O系统以及有关形成的水合物结构的信息。在本研究中,我们报告了水对通过单晶X射线衍射技术和拉曼光谱法研究的基于LiTDI甘醇二甲醚的电解质的影响。我们发现,4,5-二氰基咪唑酸锂-甘醇二甲氧基锂溶剂暴露在潮湿的空气中会产生水解产物,这是由于将水分子逐步添加到锂配位球中而引起的。在LiTDI–H 2中,几种结构基序已被区分为优选的协调模式。O系统。大量可用的醚氧供体中心水分子引起离子接触对的解离和由冠醚稳定的阳离子物种的聚集。低的O:Li摩尔比导致形成LiTDI-甘醇-水溶剂化物和LiTDI水合物。空气稳定的LiTDI三水合物包含由与TDI的咪唑氮配位的锂阳离子形成的离子对。通过腈基和含水分子配位的锂阳离子是构成式[Li(H 2 O)2 TDI] 2的二聚体的基本基序,存在于聚集的[Li(H 2 O)TDI] n中链构成一水合物的结构。已经证明发现的基序在LiTDI的固体和熔融水合系统中均会发生。它们将有助于进行分子动力学计算并获得有关如何基于杂环阴离子在水合和液体含水电解质中操纵Li +溶剂化鞘层结构的信息。
更新日期:2018-02-01
中文翻译:

4,5-二氰基咪唑酸锂水解的快照–甘醇二甲醚。水分子对锂离子电池电解质聚集过程的影响
尽管4,5-二氰基-2-(三氟甲基)咪唑锂盐(LiTDI)在非质子传递溶剂(如甘醇二甲酸酯或碳酸酯)中表现出几个有趣的特征,但在暴露于水后其结构重排知之甚少。由于LiTDI盐已被证实是能够抑制基于LiPF 6的电解质降解的有效水分清除剂,因此需要全面了解LiTDI–H 2中的配位模式理想的是O系统以及有关形成的水合物结构的信息。在本研究中,我们报告了水对通过单晶X射线衍射技术和拉曼光谱法研究的基于LiTDI甘醇二甲醚的电解质的影响。我们发现,4,5-二氰基咪唑酸锂-甘醇二甲氧基锂溶剂暴露在潮湿的空气中会产生水解产物,这是由于将水分子逐步添加到锂配位球中而引起的。在LiTDI–H 2中,几种结构基序已被区分为优选的协调模式。O系统。大量可用的醚氧供体中心水分子引起离子接触对的解离和由冠醚稳定的阳离子物种的聚集。低的O:Li摩尔比导致形成LiTDI-甘醇-水溶剂化物和LiTDI水合物。空气稳定的LiTDI三水合物包含由与TDI的咪唑氮配位的锂阳离子形成的离子对。通过腈基和含水分子配位的锂阳离子是构成式[Li(H 2 O)2 TDI] 2的二聚体的基本基序,存在于聚集的[Li(H 2 O)TDI] n中链构成一水合物的结构。已经证明发现的基序在LiTDI的固体和熔融水合系统中均会发生。它们将有助于进行分子动力学计算并获得有关如何基于杂环阴离子在水合和液体含水电解质中操纵Li +溶剂化鞘层结构的信息。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号