当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Disulfide Sensitivity in the Env Protein Underlies Lytic Inactivation of HIV-1 by Peptide Triazole Thiols
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2015-10-22 00:00:00 , DOI: 10.1021/acschembio.5b00381
Lauren D. Bailey 1 , Ramalingam Venkat Kalyana Sundaram 1, 2 , Huiyuan Li 1 , Caitlin Duffy 1 , Rachna Aneja 1 , Arangassery Rosemary Bastian 3 , Andrew P. Holmes 1 , Kantharaju Kamanna 1 , Adel A. Rashad 1 , Irwin Chaiken 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2015-10-22 00:00:00 , DOI: 10.1021/acschembio.5b00381
Lauren D. Bailey 1 , Ramalingam Venkat Kalyana Sundaram 1, 2 , Huiyuan Li 1 , Caitlin Duffy 1 , Rachna Aneja 1 , Arangassery Rosemary Bastian 3 , Andrew P. Holmes 1 , Kantharaju Kamanna 1 , Adel A. Rashad 1 , Irwin Chaiken 1
Affiliation
|
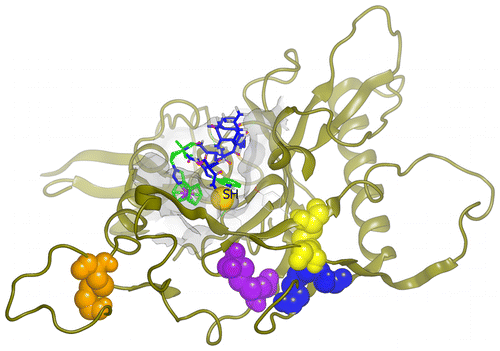
We investigated the mode of action underlying lytic inactivation of HIV-1 virions by peptide triazole thiol (PTT), in particular the relationship between gp120 disulfides and the C-terminal cysteine-SH required for virolysis. Obligate PTT dimer obtained by PTT SH cross-linking and PTTs with serially truncated linkers between pharmacophore isoleucine–ferrocenyltriazole-proline–tryptophan and cysteine-SH were synthesized. PTT variants showed loss of lytic activity but not binding and infection inhibition upon SH blockade. A disproportionate loss of lysis activity vs binding and infection inhibition was observed upon linker truncation. Molecular docking of PTT onto gp120 argued that, with sufficient linker length, the peptide SH could approach and disrupt several alternative gp120 disulfides. Inhibition of lysis by gp120 mAb 2G12, which binds at the base of the V3 loop, as well as disulfide mutational effects, argued that PTT-induced disruption of the gp120 disulfide cluster at the base of the V3 loop is an important step in lytic inactivation of HIV-1. Further, PTT-induced lysis was enhanced after treating virus with reducing agents dithiothreitol and tris (2-carboxyethyl)phosphine. Overall, the results are consistent with the view that the binding of PTT positions the peptide SH group to interfere with conserved disulfides clustered proximal to the CD4 binding site in gp120, leading to disulfide exchange in gp120 and possibly gp41, rearrangement of the Env spike, and ultimately disruption of the viral membrane. The dependence of lysis activity on thiol–disulfide interaction may be related to intrinsic disulfide exchange susceptibility in gp120 that has been reported previously to play a role in HIV-1 cell infection.
中文翻译:

Env蛋白中的二硫键敏感性是肽三唑硫醇对HIV-1的裂解作用的基础。
我们研究了通过肽三唑硫醇(PTT)对HIV-1病毒体进行裂解失活的作用方式,特别是gp120二硫化物与C端半胱氨酸-SH进行病毒水解之间的关系。合成了通过PTT SH交联得到的专性PTT二聚体,以及在药效团异亮氨酸-二茂铁基三唑-脯氨酸-色氨酸和半胱氨酸-SH之间具有连续截短的接头的PTT。PTT变体显示出裂解活性的丧失,但没有结合并且在SH阻断时没有感染抑制作用。接头截短后观察到裂解活性相对于结合和感染抑制不成比例地损失。PTT与gp120的分子对接表明,具有足够的接头长度,肽SH可以接近并破坏几种gp120二硫化物。gp120 mAb 2G12抑制裂解,它结合在V3环的底部以及二硫键的突变效应,认为PTT诱导的在V3环的底部gp120二硫键簇的破坏是HIV-1裂解失活的重要步骤。此外,用还原剂二硫苏糖醇和三(2-羧乙基)膦处理病毒后,PTT诱导的裂解作用增强。总体而言,该结果与以下观点一致:PTT的结合使肽SH基团干扰了gp120中CD4结合位点附近聚集的保守二硫键,从而导致gp120中可能进行二硫键交换,可能导致gp41发生,Env峰的重排,并最终破坏病毒膜。
更新日期:2015-10-22
中文翻译:

Env蛋白中的二硫键敏感性是肽三唑硫醇对HIV-1的裂解作用的基础。
我们研究了通过肽三唑硫醇(PTT)对HIV-1病毒体进行裂解失活的作用方式,特别是gp120二硫化物与C端半胱氨酸-SH进行病毒水解之间的关系。合成了通过PTT SH交联得到的专性PTT二聚体,以及在药效团异亮氨酸-二茂铁基三唑-脯氨酸-色氨酸和半胱氨酸-SH之间具有连续截短的接头的PTT。PTT变体显示出裂解活性的丧失,但没有结合并且在SH阻断时没有感染抑制作用。接头截短后观察到裂解活性相对于结合和感染抑制不成比例地损失。PTT与gp120的分子对接表明,具有足够的接头长度,肽SH可以接近并破坏几种gp120二硫化物。gp120 mAb 2G12抑制裂解,它结合在V3环的底部以及二硫键的突变效应,认为PTT诱导的在V3环的底部gp120二硫键簇的破坏是HIV-1裂解失活的重要步骤。此外,用还原剂二硫苏糖醇和三(2-羧乙基)膦处理病毒后,PTT诱导的裂解作用增强。总体而言,该结果与以下观点一致:PTT的结合使肽SH基团干扰了gp120中CD4结合位点附近聚集的保守二硫键,从而导致gp120中可能进行二硫键交换,可能导致gp41发生,Env峰的重排,并最终破坏病毒膜。







































 京公网安备 11010802027423号
京公网安备 11010802027423号