当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron(II)-Catalyzed Hydrogenation of Acetophenone with a Chiral, Pyridine-Based PNP Pincer Ligand: Support for an Outer-Sphere Mechanism
Organometallics ( IF 2.5 ) Pub Date : 2018-01-24 00:00:00 , DOI: 10.1021/acs.organomet.7b00816 Raffael Huber 1 , Alessandro Passera 1 , Antonio Mezzetti 1
Organometallics ( IF 2.5 ) Pub Date : 2018-01-24 00:00:00 , DOI: 10.1021/acs.organomet.7b00816 Raffael Huber 1 , Alessandro Passera 1 , Antonio Mezzetti 1
Affiliation
|
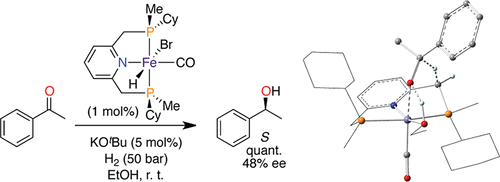
We report here the tridentate, P-stereogenic, C2-symmetric PNP pincer ligand (SP,SP)-2,6-bis((cyclohexyl(methyl)phosphanyl)methyl)pyridine (1a) and its iron(II) complexes [FeBr2(CO)(1a)] (2a), [FeHBr(CO)(1a)] (3a), and [FeH2(CO)(1a)] (4a). In the presence of base, bromocarbonylhydride 3a catalyzes the hydrogenation of acetophenone to (S)-1-phenylethanol with 48% ee. The transition states of the enantiodetermining transfer of hydride from 3a to the carbonyl group of acetophenone were studied by density functional theory (DFT) with a full conformational analysis of the PNP ligand for the three different mechanistic models recently proposed for a related achiral catalyst. The DFT calculations show that the outer-sphere monohydride mechanism originally proposed by Milstein reproduces the experimentally observed sense of induction (S) and enantioselectivity, whereas the dihydride and inner-sphere pathways predict the formation of the R enantiomer.
中文翻译:

铁(II)催化的手性,吡啶基PNP钳配体苯乙酮的加氢:支持外层机制
我们在这里报告的三齿,P-stereogenic,C 2对称PNP钳配体(S P,S P)-2,6-双((环己基(甲基)膦基)甲基)吡啶(1a)及其铁(II)配合物[FeBr 2(CO)(1a)](2a),[FeHBr(CO)(1a)](3a)和[FeH 2(CO)(1a)](4a)。在碱的存在下,溴羰基氢化物3a催化苯乙酮氢化为ee为48%的(S)-1-苯基乙醇。对映体确定氢化物从中转移的过渡态通过密度泛函理论(DFT)研究了苯乙酮的3a羰基,并针对最近提出的有关非手性催化剂的三种不同机理模型对PNP配体进行了完整的构象分析。DFT计算表明,Milstein最初提出的外球一氢化物机理再现了实验观察到的诱导感(S)和对映体选择性,而二氢化物和内球途径预测了R对映体的形成。
更新日期:2018-01-24
中文翻译:

铁(II)催化的手性,吡啶基PNP钳配体苯乙酮的加氢:支持外层机制
我们在这里报告的三齿,P-stereogenic,C 2对称PNP钳配体(S P,S P)-2,6-双((环己基(甲基)膦基)甲基)吡啶(1a)及其铁(II)配合物[FeBr 2(CO)(1a)](2a),[FeHBr(CO)(1a)](3a)和[FeH 2(CO)(1a)](4a)。在碱的存在下,溴羰基氢化物3a催化苯乙酮氢化为ee为48%的(S)-1-苯基乙醇。对映体确定氢化物从中转移的过渡态通过密度泛函理论(DFT)研究了苯乙酮的3a羰基,并针对最近提出的有关非手性催化剂的三种不同机理模型对PNP配体进行了完整的构象分析。DFT计算表明,Milstein最初提出的外球一氢化物机理再现了实验观察到的诱导感(S)和对映体选择性,而二氢化物和内球途径预测了R对映体的形成。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号