当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Redox-Neutral α-C–H Functionalization of Pyrrolidin-3-ol
Organic Letters ( IF 4.9 ) Pub Date : 2018-01-24 00:00:00 , DOI: 10.1021/acs.orglett.7b03807 Cheng-Bo Yi 1 , Zhi-Ying She 1 , Yong-Feng Cheng 1 , Jin Qu 1
Organic Letters ( IF 4.9 ) Pub Date : 2018-01-24 00:00:00 , DOI: 10.1021/acs.orglett.7b03807 Cheng-Bo Yi 1 , Zhi-Ying She 1 , Yong-Feng Cheng 1 , Jin Qu 1
Affiliation
|
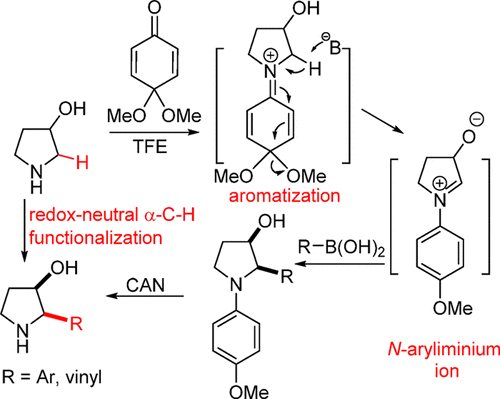
A redox-neutral α-C–H oxygenation of commercially available pyrrolidin-3-ol with a monoprotected p-quinone generated an N-aryliminium ion intermediate, which reacted in situ with boronic acid nucleophiles to produce a series of cis-2-substituted pyrrolidin-3-ols. With this strategy, 8-epi-(−)-lentiginosine was synthesized from (3R,4R)-pyrrolidine-3,4-diol in three steps.
中文翻译:

Pyrrolidin-3-ol的氧化还原中性α-C–H官能化
市售吡咯烷-3-醇与单保护的对苯醌的氧化还原中性α-CH氧合生成N-芳基亚胺离子中间体,该中间体与硼酸亲核试剂原位反应生成一系列顺式-2-取代的吡咯烷-3-醇。通过这种策略,通过三个步骤从(3R,4R)-吡咯烷-3,4-二醇合成了8- epi -(-)-lentiginosine 。
更新日期:2018-01-24
中文翻译:

Pyrrolidin-3-ol的氧化还原中性α-C–H官能化
市售吡咯烷-3-醇与单保护的对苯醌的氧化还原中性α-CH氧合生成N-芳基亚胺离子中间体,该中间体与硼酸亲核试剂原位反应生成一系列顺式-2-取代的吡咯烷-3-醇。通过这种策略,通过三个步骤从(3R,4R)-吡咯烷-3,4-二醇合成了8- epi -(-)-lentiginosine 。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号