npj Parkinson's Disease ( IF 6.7 ) Pub Date : 2018-01-24 , DOI: 10.1038/s41531-017-0040-2 David G. Standaert , James T. Boyd , Per Odin , Weining Z. Robieson , Jorge Zamudio , Krai Chatamra
|
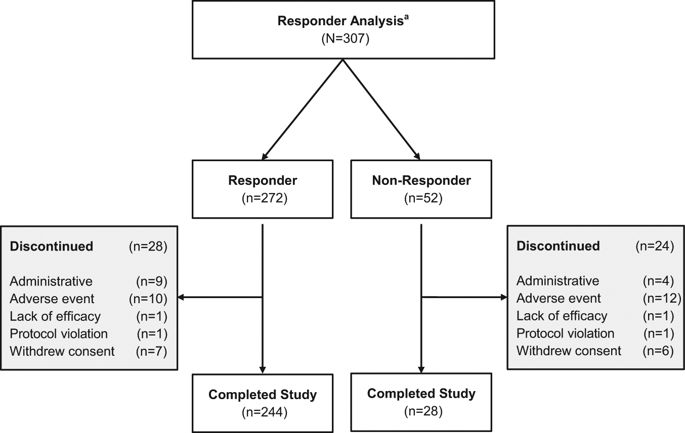
Levodopa-carbidopa intestinal gel (LCIG, carbidopa-levodopa enteral suspension in the United States) is a treatment option for advanced Parkinson’s disease (PD) patients with motor fluctuations. The objective of this investigation was to identify the baseline characteristics predictive of treatment response, measured by improvement in motor symptom severity, in advanced PD patients treated with LCIG during a 54-week, open-label phase 3 study. Patients with ≥1 h improvement from baseline in “Off” time were categorized as “Responders”; whereas those with <1 h improvement, any worsening, or no post-baseline assessment were “Non-Responders”. A subgroup of Responders with ≥3 h improvement in “Off” time was also examined; this subgroup was identified as “Robust Responders”. Baseline demographics and disease characteristics were analyzed and their predictive relationship to change from baseline in normalized “Off” time was assessed. Out of the 324 patients included in the analysis, 272 (84.0%) were categorized as Responders and 52 (16.0%) were Non-Responders. A majority of patients (65.7%) had ≥3 h improvement in “Off” time. In general, baseline characteristics were similar between Non-responders, Responders, and the subgroup of Robust Responders. A conditional tree-structured regression analysis identified baseline “Off” time as the only factor that had significant effect on Responder and Robust Responder status. The safety profile of LCIG was similar between patient groups. Overall, this analysis showed that 84% of LCIG-treated advanced PD patients had ≥1 h improvement in “Off” time and the number-needed-to-treat to observe one patient responder was 1.19 patients. Notably, Responders and Robust Responders to LCIG were observed across the range of baseline demographics and clinical characteristics examined.
中文翻译:

左旋多巴-卡比多巴肠凝胶患者反应者特征的系统评价
左旋多巴-卡比多巴肠凝胶(在美国为LCIG,卡比多巴-左旋多巴肠混悬液)是患有运动波动的晚期帕金森氏病(PD)患者的治疗选择。这项研究的目的是确定在54周的开放标签3期研究中,接受LCIG治疗的晚期PD患者的基线特征,这些基线特征可预测运动反应的严重程度(通过运动症状严重程度的改善来衡量)。在“关闭”时间内与基线相比改善≥1 h的患者被归类为“响应者”;而改善时间少于1小时,恶化或未进行基线后评估的患者为“无反应者”。还检查了“关闭”时间改善≥3h的响应者亚组。该子组被称为“健壮的响应者”。分析了基线人口统计资料和疾病特征,并评估了它们在归一化“关闭”时间内相对于基线变化的预测关系。在分析的324位患者中,有272位(84.0%)被归类为响应者,而52位(16.0%)被归为非响应者。大多数患者(65.7%)的“关闭”时间改善了≥3小时。通常,“无响应者”,“响应者”和“稳健响应者”子组之间的基线特征相似。有条件的树状结构回归分析确定基线“关闭”时间是对响应者和鲁棒响应者状态产生重大影响的唯一因素。患者组之间LCIG的安全性相似。全面的,该分析表明,在接受LCIG治疗的晚期PD患者中,有84%的“关闭”时间改善了≥1 h,观察一名患者反应者所需的治疗次数为1.19患者。值得注意的是,在基线人口统计学和临床特征范围内观察到了对LCIG的反应者和稳健的反应者。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号